Penicillin binding protein 5 affects cell diameter, contour, and morphology of Escherichia coli
- PMID: 10692378
- PMCID: PMC94470
- DOI: 10.1128/JB.182.6.1714-1721.2000
Penicillin binding protein 5 affects cell diameter, contour, and morphology of Escherichia coli
Abstract
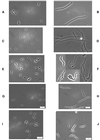
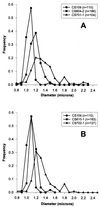
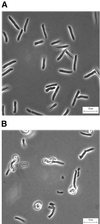
Although general physiological functions have been ascribed to the high-molecular-weight penicillin binding proteins (PBPs) of Escherichia coli, the low-molecular-weight PBPs have no well-defined biological roles. When we examined the morphology of a set of E. coli mutants lacking multiple PBPs, we observed that strains expressing active PBP 5 produced cells of normal shape, while mutants lacking PBP 5 produced cells with altered diameters, contours, and topological features. These morphological effects were visible in untreated cells, but the defects were exacerbated in cells forced to filament by inactivation of PBP 3 or FtsZ. After filamentation, cellular diameter varied erratically along the length of individual filaments and many filaments exhibited extensive branching. Also, in general, the mean diameter of cells lacking PBP 5 was significantly increased compared to that of cells from isogenic strains expressing active PBP 5. Expression of cloned PBP 5 reversed the effects observed in DeltadacA mutants. Although deletion of PBP 5 was required for these phenotypes, the absence of additional PBPs magnified the effects. The greatest morphological alterations required that at least three PBPs in addition to PBP 5 be deleted from a single strain. In the extreme cases in which six or seven PBPs were deleted from a single mutant, cells and cell filaments expressing PBP 5 retained a normal morphology but cells and filaments lacking PBP 5 were aberrant. In no case did mutation of another PBP produce the same drastic morphological effects. We conclude that among the low-molecular-weight PBPs, PBP 5 plays a principle role in determining cell diameter, surface uniformity, and overall topology of the peptidoglycan sacculus.
Figures

References
-
- Åkerlund T, Nordström K, Bernander R. Branched Escherichia coli cells. Mol Microbiol. 1993;10:849–858. - PubMed
-
- Edwards D H, Donachie W D. Construction of a triple deletion of penicillin-binding proteins 4, 5, and 6 in Escherichia coli. In: de Pedro M A, Höltje J-V, Löffelhardt W, editors. Bacterial growth and lysis. New York, N.Y: Plenum Press; 1993. pp. 369–374.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

