Mechanisms of AVP-induced glucagon release in clonal alpha-cells in-R1-G9: involvement of Ca(2+)-dependent and -independent pathways
- PMID: 10694231
- PMCID: PMC1571828
- DOI: 10.1038/sj.bjp.0703037
Mechanisms of AVP-induced glucagon release in clonal alpha-cells in-R1-G9: involvement of Ca(2+)-dependent and -independent pathways
Abstract
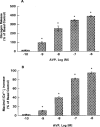
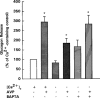
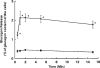
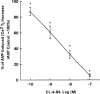
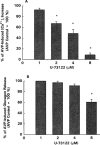
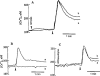
1. The mechanisms underlying AVP-induced increase in [Ca(2+)](i) and glucagon release in clonal alpha-cells In-R1-G9 were investigated. 2. AVP increased [Ca(2+)](i) and glucagon release in a concentration-dependent manner. After the administration of AVP, glucagon was released within 30 s, quickly reached the maximum within 2 min, and maintained a steady-state concentration for at least 15 min. 3. In Ca(2+)-containing medium, AVP increased [Ca(2+)](i) in a biphasic pattern; a peak followed by a sustained plateau. In Ca(2+)-free medium, the Ca(2+) response to AVP became monophasic with lower amplitude and no plateau. Both the basal and AVP-induced glucagon releases were lower in the absence than in the presence of extracellular Ca(2+). When [Ca(2+)](i) was stringently deprived by BAPTA, a Ca(2+) chelator, AVP still significantly increased glucagon release. 4. Pretreatment with thapsigargin, a microsomal Ca(2+) ATPase inhibitor, abolished both the Ca(2+) peak and sustained plateau. 5.AVP increased intracellular concentration of IP(3). 6. U-73122 (8 microM), a phospholipase C inhibitor, abolished AVP-induced increases in [Ca(2+)](i), but only reduced AVP-induced glucagon release by 39%. 7. Pretreatment with nimodipine, an L-type Ca(2+) channel blocker failed to alter AVP-induced glucagon release or increase in [Ca(2+)](i). 8. The results suggest that AVP causes glucagon release through both Ca(2+)-dependent and -independent pathways. For the Ca(2+)-dependent pathway, the G(q) protein activates phospholipase C, which catalyzes the formation of IP(3). IP(3) induces Ca(2+) release from the endoplasmic reticulum, which, in turn, triggers Ca(2+) influx. Both Ca(2+) release and Ca(2+) influx may contribute to AVP-induced glucagon release.
Figures

References
-
- BLEASDALE J.E., FISHER S.K. Use of U-73122 as an inhibitor of phospholipase C-dependent processes. Neuroprotocols. 1993;3:125–133.
-
- BLEASDALE J.E., THAKUR N.R., GREMBAN R.S., BUNDY G.L., FITZPATRICK F.A., SMITH R.J., BUNTING S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J. Pharmacol. Exp. Ther. 1990;225:756–768. - PubMed
-
- BODE H.P., YULE D.I., FEHMANN H.C., GOKE B., WILLIAMS J.A. Spontaneous calcium oscillations in clonal endocrine pancreatic glucagon-secreting cells. Biochem. Biophys. Res. Commun. 1994;205:435–440. - PubMed
-
- CHEN T.H., LEE B., HSU W.H. Arginine vasopressin-stimulated insulin secretion and elevation of intracellular Ca++ concentration in rat insulinoma cells: Influences of a phospholipase C inhibitor 1-[6-[[17β-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione (U-73122) and a phospholipase A2 inhibitor N-(p-Amylcinnamoyl)anthranilic acid. J. Pharmacol. Exp. Ther. 1994;270:900–904. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

