Modulation of N-type calcium channel activity by G-proteins and protein kinase C
- PMID: 10694257
- PMCID: PMC2217210
- DOI: 10.1085/jgp.115.3.277
Modulation of N-type calcium channel activity by G-proteins and protein kinase C
Abstract
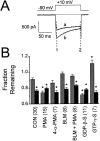
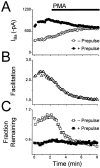
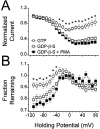
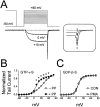
N-type voltage-gated calcium channel activity in rat superior cervical ganglion neurons is modulated by a variety of pathways. Activation of heterotrimeric G-proteins reduces whole-cell current amplitude, whereas phosphorylation by protein kinase C leads to an increase in current amplitude. It has been proposed that these two distinct pathways converge on the channel's pore-forming alpha(1B) subunit, such that the actions of one pathway can preclude those of the other. In this study, we have characterized further the actions of PKC on whole-cell barium currents in neonatal rat superior cervical ganglion neurons. We first examined whether the effects of G-protein-mediated inhibition and phosphorylation by PKC are mutually exclusive. G-proteins were activated by including 0.4 mM GTP or 0.1 mM GTP-gamma-S in the pipette, and PKC was activated by bath application of 500 nM phorbol 12-myristate 13-acetate (PMA). We found that activated PKC was unable to reverse GTP-gamma-S-induced inhibition unless prepulses were applied, indicating that reversal of inhibition by phosphorylation appears to occur only after dissociation of the G-protein from the channel. Once inhibition was relieved, activation of PKC was sufficient to prevent reinhibition of current by G-proteins, indicating that under phosphorylating conditions, channels are resistant to G-protein-mediated modulation. We then examined what effect, if any, phosphorylation by PKC has on N-type barium currents beyond antagonizing G-protein-mediated inhibition. We found that, although G-protein activation significantly affected peak current amplitude, fast inactivation, holding-potential-dependent inactivation, and voltage-dependent activation, when G-protein activation was minimized by dialysis of the cytoplasm with 0.1 mM GDP-beta-S, these parameters were not affected by bath application of PMA. These results indicate that, under our recording conditions, phosphorylation by PKC has no effect on whole-cell N-type currents, other than preventing inhibition by G-proteins.
Figures





Comment in
-
Modulating modulation.J Gen Physiol. 2000 Mar;115(3):273-5. doi: 10.1085/jgp.115.3.273. J Gen Physiol. 2000. PMID: 10694256 Free PMC article. Review. No abstract available.
References
-
- Bean B.P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. - PubMed
-
- de Leon M., Wang Y., Jones L., Perez-Reyes E., Wei X., Soong T.W., Snutch T.P., Yue D.T. Essential Ca(2+)-binding motif for Ca(2+)-sensitive inactivation of L-type Ca2+ channels. Science. 1995;270:1502–1506. - PubMed
-
- Erlich I., Elmslie K.S. Neurotransmitters acting via different G proteins inhibit N-type calcium current by an identical mechanism in rat sympathetic neurons. J. Neurophysiol. 1995;74:2251–2257. - PubMed

