Activation of Drosophila sodium channels promotes modification by deltamethrin. Reductions in affinity caused by knock-down resistance mutations
- PMID: 10694259
- PMCID: PMC2217214
- DOI: 10.1085/jgp.115.3.305
Activation of Drosophila sodium channels promotes modification by deltamethrin. Reductions in affinity caused by knock-down resistance mutations
Abstract
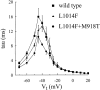
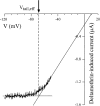
kdr and super-kdr are mutations in houseflies and other insects that confer 30- and 500-fold resistance to the pyrethroid deltamethrin. They correspond to single (L1014F) and double (L1014F+M918T) mutations in segment IIS6 and linker II(S4-S5) of Na channels. We expressed Drosophila para Na channels with and without these mutations and characterized their modification by deltamethrin. All wild-type channels can be modified by <10 nM deltamethrin, but high affinity binding requires channel opening: (a) modification is promoted more by trains of brief depolarizations than by a single long depolarization, (b) the voltage dependence of modification parallels that of channel opening, and (c) modification is promoted by toxin II from Anemonia sulcata, which slows inactivation. The mutations reduce channel opening by enhancing closed-state inactivation. In addition, these mutations reduce the affinity for open channels by 20- and 100-fold, respectively. Deltamethrin inhibits channel closing and the mutations reduce the time that channels remain open once drug has bound. The super-kdr mutations effectively reduce the number of deltamethrin binding sites per channel from two to one. Thus, the mutations reduce both the potency and efficacy of insecticide action.
Figures




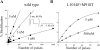

Similar articles
-
Sensitivity of the Drosophila para sodium channel to DDT is not lowered by the super-kdr mutation M918T on the IIS4-S5 linker that profoundly reduces sensitivity to permethrin and deltamethrin.FEBS Lett. 2005 Nov 21;579(28):6317-25. doi: 10.1016/j.febslet.2005.09.096. Epub 2005 Oct 21. FEBS Lett. 2005. PMID: 16263118
-
The molecular interactions of pyrethroid insecticides with insect and mammalian sodium channels.Pest Manag Sci. 2001 Oct;57(10):877-88. doi: 10.1002/ps.392. Pest Manag Sci. 2001. PMID: 11695180 Review.
-
Mutations of the para sodium channel of Drosophila melanogaster identify putative binding sites for pyrethroids.Mol Pharmacol. 2003 Oct;64(4):914-22. doi: 10.1124/mol.64.4.914. Mol Pharmacol. 2003. PMID: 14500748
-
Differential state-dependent modification of inactivation-deficient Nav1.6 sodium channels by the pyrethroid insecticides S-bioallethrin, tefluthrin and deltamethrin.Neurotoxicology. 2012 Jun;33(3):384-90. doi: 10.1016/j.neuro.2012.03.007. Epub 2012 Mar 28. Neurotoxicology. 2012. PMID: 22465659 Free PMC article.
-
Evolution of resistance to pyrethroid insecticides in Musca domestica.Pest Manag Sci. 2017 Apr;73(4):716-722. doi: 10.1002/ps.4328. Epub 2016 Jul 13. Pest Manag Sci. 2017. PMID: 27241012 Review.
Cited by
-
Mutations of two acidic residues at the cytoplasmic end of segment IIIS6 of an insect sodium channel have distinct effects on pyrethroid resistance.Insect Biochem Mol Biol. 2017 Mar;82:1-10. doi: 10.1016/j.ibmb.2017.01.007. Epub 2017 Jan 20. Insect Biochem Mol Biol. 2017. PMID: 28111191 Free PMC article.
-
A negative charge in transmembrane segment 1 of domain II of the cockroach sodium channel is critical for channel gating and action of pyrethroid insecticides.Toxicol Appl Pharmacol. 2010 Aug 15;247(1):53-9. doi: 10.1016/j.taap.2010.05.016. Epub 2010 Jun 1. Toxicol Appl Pharmacol. 2010. PMID: 20561903 Free PMC article.
-
Characterization of the honeybee AmNaV1 channel and tools to assess the toxicity of insecticides.Sci Rep. 2015 Jul 23;5:12475. doi: 10.1038/srep12475. Sci Rep. 2015. PMID: 26202396 Free PMC article.
-
First report on co-occurrence knockdown resistance mutations and susceptibility to beta-cypermethrin in Anopheles sinensis from Jiangsu Province, China.PLoS One. 2012;7(1):e29242. doi: 10.1371/journal.pone.0029242. Epub 2012 Jan 17. PLoS One. 2012. PMID: 22272229 Free PMC article.
-
Evolutionary origin and distribution of amino acid mutations associated with resistance to sodium channel modulators in onion thrips, Thrips tabaci.Sci Rep. 2024 Feb 15;14(1):3792. doi: 10.1038/s41598-024-54443-9. Sci Rep. 2024. PMID: 38360913 Free PMC article.
References
-
- Bloomquist J.R. Neuroreceptor mechanisms in pyrethroid mode of action and resistance. In: Roe R.M., Kuhr R.J., editors. Reviews in Pesticide Toxicology. Toxicology Communications Inc; Raleigh, NC: 1993. pp. 185–230.
-
- Bloomquist J.R. Ion channels as targets for insecticides. Annu. Rev. Entomol. 1996;41:163–190. - PubMed
-
- Catterall W.A. Cellular and molecular biology of voltage-gated sodium channels. Physiol. Rev. 1992;72:S15–S48. - PubMed
-
- Cestele S., Qu Y., Rogers J.C., Rochat H., Scheuer T., Catterall W.A. Voltage sensor-trappingenhanced activation of sodium channels by beta-scorpion toxin bound to the S3–S4 loop in domain II. Neuron. 1998;21:919–931. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

