Peroxisome proliferator-activated receptors in the cardiovascular system
- PMID: 10696077
- PMCID: PMC1571927
- DOI: 10.1038/sj.bjp.0703149
Peroxisome proliferator-activated receptors in the cardiovascular system
Abstract
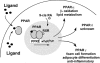
Peroxisome proliferator-activated receptor (PPAR)s are a family of three nuclear hormone receptors, PPARalpha, -delta, and -gamma, which are members of the steriod receptor superfamily. The first member of the family (PPARalpha) was originally discovered as the mediator by which a number of xenobiotic drugs cause peroxisome proliferation in the liver. Defined functions for all these receptors, until recently, mainly concerned their ability to regulate energy balance, with PPARalpha being involved in beta-oxidation pathways, and PPARgamma in the differentiation of adipocytes. Little is known about the functions of PPARdelta, though it is the most ubiquitously expressed. Since their discovery, PPARs have been shown to be expressed in monocytes/macrophages, the heart, vascular smooth muscle cells, endothelial cells, and in atherosclerotic lesions. Furthermore, PPARs can be activated by a vast number of compounds including synthetic drugs, of the clofibrate, and anti-diabetic thiazoldinedione classes, polyunsaturated fatty acids, and a number of eicosanoids, including prostaglandins, lipoxygenase products, and oxidized low density lipoprotein. This review will aim to introduce the field of PPAR nuclear hormone receptors, and discuss the discovery and actions of PPARs in the cardiovascular system, as well as the source of potential ligands.
Figures

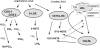
References
-
- AUBOEUF D., RIEUSSET J., FAJAS L., VALLIER P., FRERING V., RIOU J.P., STAELS B., AUWERX J., LAVILLE M., VIDAL H. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes. 1997;46:1319–1327. - PubMed
-
- BAKER C.S., HALL R.J., EVANS T.J., POMERANCE A., MACLOUF J., CREMINON C., YACOUB M.H., POLAK J.M. Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arteriscler. Thromb. Vasc. Biol. 1999;19:646–655. - PubMed
-
- BENZ D.J., MOL M., EZAKI M., MORI-ITO N., ZELAN I., MIYANOHARA A., FRIEDMAN T., PARTHASARATHY S., STEINBERG D., WITZTUM J.L. Enhanced levels of lipoperoxides in low density lipoprotein incubated with murine fibroblasts expressing high levels of human 15-lipoxygenase. J. Biol. Chem. 1995;270:5191–5197. - PubMed
-
- BERLINER J.A., HEINECKE J.W. The role of oxidized lipoproteins in atherogenesis. Free Radical Biol. Med. 1996;20:707–727. - PubMed
-
- BISHOP-BAILEY D., HLA T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Δ12,14 prostaglandin J2. J. Biol. Chem. 1999;274:17042–17048. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

