Electrophysiological effects of protopine in cardiac myocytes: inhibition of multiple cation channel currents
- PMID: 10696087
- PMCID: PMC1571915
- DOI: 10.1038/sj.bjp.0703132
Electrophysiological effects of protopine in cardiac myocytes: inhibition of multiple cation channel currents
Abstract
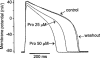
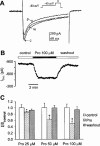
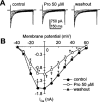
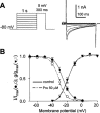
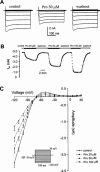
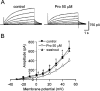
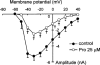
Protopine (Pro) from Corydalis tubers has been shown to have multiple actions on cardiovascular system, including anti-arrhythmic, anti-hypertensive and negative inotropic effects. Although it was thought that Pro exerts its actions through blocking Ca(2+) currents, the electrophysiological profile of Pro is unclear. The aim of this study is to elucidate the ionic mechanisms of Pro effects in the heart. In single isolated ventricular myocytes from guinea-pig, extracellular application of Pro markedly and reversibly abbreviates action potential duration, and decreases the rate of upstroke (dV/dt)(max), amplitude and overshoot of action potential in a dose-dependent manner. Additionally, it produces a slight, but significant hyperpolarization of the resting membrane potential. Pro at 25, 50 and 100 microM reduces L-type Ca(2+) current (I(Ca,L)) amplitude to 89.1, 61.9 and 45.8% of control, respectively, and significantly slows the decay kinetics of I(Ca,L) at higher concentration. The steady state inactivation of I(Ca,L) is shifted negatively by 5.9 - 7.0 mV (at 50 - 100 microM Pro), whereas the voltage-dependent activation of I(Ca,L) remains unchanged. In contrast, Pro at 100 microM has no evident effects on T-type Ca(2+) current (I(Ca,T)). In the presence of Pro, both the inward rectifier (I(K1)) and delayed rectifier (I(K)) potassium currents are variably inhibited, depending on Pro concentrations. Sodium current (I(Na)), recorded in low [Na(+)](o) (40 mM) solution, is more potently suppressed by Pro. At 25 microM, Pro significantly attenuated I(Na) at most of the test voltages (-60 approximately +40 mV, with a 53% reduction at -30 mV. Thus, Pro is not a selective Ca(2+) channel antagonist. Rather, it acts as a promiscuous inhibitor of cation channel currents including I(Ca,L), I(K), I(K1) as well as I(Na). These findings may provide some mechanistic explanations for the therapeutic actions of Pro in the heart.
Figures
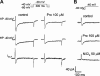
References
-
- BERLIN J.R., CANNELL M.B., LEDERER W.J. Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circ. Res. 1989;65:115–126. - PubMed
-
- BURTSEV V.N., DORMIDONTOV E.N., SALIAEV V.N. Prevention of ventricular fibrillation with the aid of protopine in animal experiments. Kardiologiia. 1978;18:76–79. - PubMed
-
- EISNER D.A., TRAFFORD A.W., DIAZ M.E., OVEREND C.L., O'NEILL S.C. The control of Ca release from the cardiac sacroplasmic reticulum: regulation versus autoregulation. J. Physiol. 1998;38:589–604. - PubMed
-
- FABIATO A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983;245:C1–C14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

