DNA replication and cell cycle in plants: learning from geminiviruses
- PMID: 10698921
- PMCID: PMC305619
- DOI: 10.1093/emboj/19.5.792
DNA replication and cell cycle in plants: learning from geminiviruses
Abstract
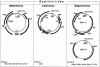
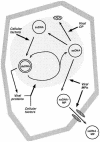
Plant cell growth and development depend on continuous cell proliferation which is restricted to small regions of the plant called meristems. Infection by geminiviruses, small DNA viruses whose replicative cycle relies on host cell factors, is excluded from those proliferating areas. Since most of the replicative factors are present, almost exclusively, in proliferating cells, geminivirus infection is believed to induce a cellular state permissive for viral DNA replication, e.g. S-phase or, at least, some specific S-phase functions. The molecular basis for this effect seems to be the interference that certain geminivirus proteins exert on the retinoblastoma-related (RBR) pathway, which analogously to that of animal cells, regulates plant cell cycle activation and G(1)-S transition. In some cases, geminiviruses induce cell proliferation and abnormal growth. Mechanisms other than sequestering plant RBR probably contribute to the multiple effects of geminivirus proteins on cellular gene expression, cell growth control and cellular DNA replication. Current efforts to understand the coupling of geminivirus DNA replication to cell cycle and growth control as well as the directions in which future research is aiming are reviewed.
Figures
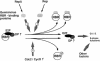
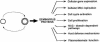
References
-
- Abouzid A.M., Barth, A. and Jeske, H. (1988) Immunogold labeling of the Abutilon mosaic virus in ultrathin sections of epoxy resin embedded leaf tissue. J. Ultrastruct. Res., 99, 39–47.
-
- Accotto G.P., Mullineaux,P.M., Brown,S.C. and Marie,D. (1993) Digitaria streak geminivirus replicative forms are abundant in S-phase nuclei of infected cells. Virology, 195, 257–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

