The importance of aquaporin water channel protein structures
- PMID: 10698922
- PMCID: PMC305620
- DOI: 10.1093/emboj/19.5.800
The importance of aquaporin water channel protein structures
Abstract
The history of the water channel and recent structural and functional analyses of aquaporins are reviewed. These ubiquitous channels are important for bacteria, plants and animals, exhibit a pronounced sequence homology and share functional as well as structural similarities. Aquaporins allow water or small specific solutes to pass unhindered, but block the passage of ions to prevent dissipation of the transmembrane potential. Besides advances in structure determination, recent experiments suggest that many of these channels are regulated by pH variations, phosphorylation and binding of auxiliary proteins.
Figures



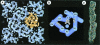

References
-
- Borgnia M., Kozono, D., Calamita, G., Nielsen, S., Maloney, P.C. and Agre, P. (1999) Functional reconstitution and characterization of E.coli aquaporin-Z. J. Mol. Biol., 291, 1169–1179. - PubMed
-
- Cahalan K.L. and Hall, J.E. (1999) pH sensitivity of MIP-induced water permeability may play a role in regulating the intrinsic fluid circulation of the lens. Biophys. J., 76, A183.
-
- Cheng A., van Hoek, A.N., Yeager, M., Verkman, A.S. and Mitra, A.K. (1997) Three-dimensional organization of a human water channel. Nature, 387, 627–630. - PubMed
-
- Cooper G.J. and Boron, W.F. (1998) Effect of PCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am. J. Physiol., 275, C1481–C1486. - PubMed
-
- Crowther R.A., Henderson, R. and Smith, J.M. (1996) MRC image processing programs. J. Struct. Biol., 116, 9–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

