Crystal structure of ribosomal protein L4 shows RNA-binding sites for ribosome incorporation and feedback control of the S10 operon
- PMID: 10698923
- PMCID: PMC305621
- DOI: 10.1093/emboj/19.5.807
Crystal structure of ribosomal protein L4 shows RNA-binding sites for ribosome incorporation and feedback control of the S10 operon
Abstract
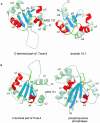
Ribosomal protein L4 resides near the peptidyl transferase center of the bacterial ribosome and may, together with rRNA and proteins L2 and L3, actively participate in the catalysis of peptide bond formation. Escherichia coli L4 is also an autogenous feedback regulator of transcription and translation of the 11 gene S10 operon. The crystal structure of L4 from Thermotoga maritima at 1.7 A resolution shows the protein with an alternating alpha/beta fold and a large disordered loop region. Two separate binding sites for RNA are discernible. The N-terminal site, responsible for binding to rRNA, consists of the disordered loop with flanking alpha-helices. The C-terminal site, a prime candidate for the interaction with the leader sequence of the S10 mRNA, involves two non-consecutive alpha-helices. The structure also suggests a C-terminal protein-binding interface, through which L4 could be interacting with protein components of the transcriptional and/or translational machineries.
Figures



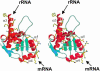

Similar articles
-
Comparative anatomy of a regulatory ribosomal protein.Biochimie. 2002 Aug;84(8):731-43. doi: 10.1016/s0300-9084(02)01410-4. Biochimie. 2002. PMID: 12457561
-
RNA-structural mimicry in Escherichia coli ribosomal protein L4-dependent regulation of the S10 operon.J Biol Chem. 2003 Jul 25;278(30):28237-45. doi: 10.1074/jbc.M302651200. Epub 2003 May 8. J Biol Chem. 2003. PMID: 12738792 Free PMC article.
-
Escherichia coli ribosomal protein L4 stimulates transcription termination at a specific site in the leader of the S10 operon independent of L4-mediated inhibition of translation.J Mol Biol. 1990 May 5;213(1):67-78. doi: 10.1016/S0022-2836(05)80122-6. J Mol Biol. 1990. PMID: 1692593
-
RNA binding strategies of ribosomal proteins.Nucleic Acids Res. 1999 Jan 15;27(2):381-8. doi: 10.1093/nar/27.2.381. Nucleic Acids Res. 1999. PMID: 9862955 Free PMC article. Review.
-
rRNA Mimicry in RNA Regulation of Gene Expression.Microbiol Spectr. 2018 Mar;6(2):10.1128/microbiolspec.rwr-0006-2017. doi: 10.1128/microbiolspec.RWR-0006-2017. Microbiol Spectr. 2018. PMID: 29546840 Free PMC article. Review.
Cited by
-
The role of disordered ribosomal protein extensions in the early steps of eubacterial 50 S ribosomal subunit assembly.Int J Mol Sci. 2009 Mar;10(3):817-834. doi: 10.3390/ijms10030817. Epub 2009 Mar 2. Int J Mol Sci. 2009. PMID: 19399222 Free PMC article. Review.
-
Ribosome Protein L4 is essential for Epstein-Barr Virus Nuclear Antigen 1 function.Proc Natl Acad Sci U S A. 2016 Feb 23;113(8):2229-34. doi: 10.1073/pnas.1525444113. Epub 2016 Feb 8. Proc Natl Acad Sci U S A. 2016. PMID: 26858444 Free PMC article.
-
Clusters of bacterial RNA polymerase are biomolecular condensates that assemble through liquid-liquid phase separation.Proc Natl Acad Sci U S A. 2020 Aug 4;117(31):18540-18549. doi: 10.1073/pnas.2005019117. Epub 2020 Jul 16. Proc Natl Acad Sci U S A. 2020. PMID: 32675239 Free PMC article.
-
Functional analysis of Saccharomyces cerevisiae ribosomal protein Rpl3p in ribosome synthesis.Nucleic Acids Res. 2007;35(12):4203-13. doi: 10.1093/nar/gkm388. Epub 2007 Jun 13. Nucleic Acids Res. 2007. PMID: 17569673 Free PMC article.
-
Deletion of L4 domains reveals insights into the importance of ribosomal protein extensions in eukaryotic ribosome assembly.RNA. 2014 Nov;20(11):1725-31. doi: 10.1261/rna.046649.114. Epub 2014 Sep 22. RNA. 2014. PMID: 25246649 Free PMC article.
References
-
- Albright R.A. and Matthews, B.W. (1998) Crystal structure of λ-Cro bound to a consensus operator at 3.0 Å resolution. J. Mol. Biol., 280, 137–151. - PubMed
-
- Arndt E., Kromer, W. and Hatakeyama, T. (1990) Organization and nucleotide sequence of a gene cluster coding for eight ribosomal proteins in the archaebacterium Halobacterium marismortui. J. Biol. Chem., 265, 3034–3039. - PubMed
-
- Bagni C., Mariottini, P., Annesi, F. and Amaldi, F. (1993) Human ribosomal protein L4: cloning and sequencing of the cDNA and primary structure of the protein. Biochim. Biophys. Acta, 1216, 475–478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

