X-ray structure of MalY from Escherichia coli: a pyridoxal 5'-phosphate-dependent enzyme acting as a modulator in mal gene expression
- PMID: 10698925
- PMCID: PMC305623
- DOI: 10.1093/emboj/19.5.831
X-ray structure of MalY from Escherichia coli: a pyridoxal 5'-phosphate-dependent enzyme acting as a modulator in mal gene expression
Abstract
MalY represents a bifunctional pyridoxal 5'-phosphate-dependent enzyme acting as a beta-cystathionase and as a repressor of the maltose regulon. Here we present the crystal structures of wild-type and A221V mutant protein. Each subunit of the MalY dimer is composed of a large pyridoxal 5'-phosphate-binding domain and a small domain similar to aminotransferases. The structural alignment with related enzymes identifies residues that are generally responsible for beta-lyase activity and depicts a unique binding mode of the pyridoxal 5'-phosphate correlated with a larger, more flexible substrate-binding pocket. In a screen for MalY mutants with reduced mal repressor properties, mutations occurred in three clusters: I, 83-84; II, 181-189 and III, 215-221, which constitute a clearly distinguished region in the MalY crystal structure far away from the cofactor. The tertiary structure of one of these mutants (A221V) demonstrates that positional rearrangements are indeed restricted to regions I, II and III. Therefore, we propose that a direct protein-protein interaction with MalT, the central transcriptional activator of the maltose system, underlies MalY-dependent repression of the maltose system.
Figures
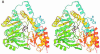
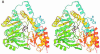






Similar articles
-
The N terminus of the Escherichia coli transcription activator MalT is the domain of interaction with MalY.J Bacteriol. 2002 Jun;184(11):3069-77. doi: 10.1128/JB.184.11.3069-3077.2002. J Bacteriol. 2002. PMID: 12003949 Free PMC article.
-
MalY of Escherichia coli is an enzyme with the activity of a beta C-S lyase (cystathionase).J Bacteriol. 1995 Sep;177(17):5035-9. doi: 10.1128/jb.177.17.5035-5039.1995. J Bacteriol. 1995. PMID: 7665481 Free PMC article.
-
Network regulation of the Escherichia coli maltose system.J Mol Microbiol Biotechnol. 2002 May;4(3):301-7. J Mol Microbiol Biotechnol. 2002. PMID: 11931562 Review.
-
Folding pathway of the pyridoxal 5'-phosphate C-S lyase MalY from Escherichia coli.Biochem J. 2005 Aug 1;389(Pt 3):885-98. doi: 10.1042/BJ20050279. Biochem J. 2005. PMID: 15823094 Free PMC article.
-
Learning new tricks from an old dog: MalT of the Escherichia coli maltose system is part of a complex regulatory network.Trends Genet. 2000 Sep;16(9):404-9. doi: 10.1016/s0168-9525(00)02086-2. Trends Genet. 2000. PMID: 10973069 Review.
Cited by
-
Crystal structure of cystalysin from Treponema denticola: a pyridoxal 5'-phosphate-dependent protein acting as a haemolytic enzyme.EMBO J. 2000 Jul 3;19(13):3168-78. doi: 10.1093/emboj/19.13.3168. EMBO J. 2000. PMID: 10880431 Free PMC article.
-
Microbial β C-S Lyases: Enzymes with Multifaceted Roles in Flavor Generation.Int J Mol Sci. 2024 Jun 11;25(12):6412. doi: 10.3390/ijms25126412. Int J Mol Sci. 2024. PMID: 38928118 Free PMC article. Review.
-
The N terminus of the Escherichia coli transcription activator MalT is the domain of interaction with MalY.J Bacteriol. 2002 Jun;184(11):3069-77. doi: 10.1128/JB.184.11.3069-3077.2002. J Bacteriol. 2002. PMID: 12003949 Free PMC article.
-
A Novel Bifunctional Amino Acid Racemase With Multiple Substrate Specificity, MalY From Lactobacillus sakei LT-13: Genome-Based Identification and Enzymological Characterization.Front Microbiol. 2018 Mar 7;9:403. doi: 10.3389/fmicb.2018.00403. eCollection 2018. Front Microbiol. 2018. PMID: 29563907 Free PMC article.
-
Crystal structure of Homo sapiens kynureninase.Biochemistry. 2007 Mar 13;46(10):2735-44. doi: 10.1021/bi0616697. Epub 2007 Feb 15. Biochemistry. 2007. PMID: 17300176 Free PMC article.
References
-
- Alexander F.W., Sandmeier, E., Mehta, P.K. and Christen, P. (1994) Evolutionary relationships among pyridoxal-5′-phosphate-dependent enzymes. Regio-specific α, β and γ families. Eur. J. Biochem., 219, 953–960. - PubMed
-
- Bouma C.L. and Roseman, S. (1996) Sugar transport by the marine chitinolytic bacterium Vibrio furnissii–molecular cloning and analysis of the glucose and N-acetylglucosamine permeases. J. Biol. Chem., 271, 33457–33467. - PubMed
-
- Brünger A.T. (1992) XPLOR (Version 3.1) Manual. Yale University Press, New Haven, CT.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

