The luminal part of the murine cytomegalovirus glycoprotein gp40 catalyzes the retention of MHC class I molecules
- PMID: 10698929
- PMCID: PMC305627
- DOI: 10.1093/emboj/19.5.870
The luminal part of the murine cytomegalovirus glycoprotein gp40 catalyzes the retention of MHC class I molecules
Abstract
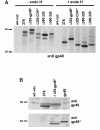
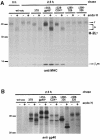
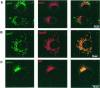
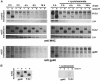
Murine cytomegalovirus (MCMV) interferes with the MHC class I pathway of antigen presentation. The type I transmembrane glycoprotein gp40, encoded by the gene m152, retains major histocompatibility complex (MHC) class I complexes in the endoplasmic reticulum (ER)-Golgi intermediate compartment (ERGIC)/cis-Golgi. These MHC class I complexes are stable, show an extended half-life and do not exchange beta(2)-microglobulin, whereas gp40 reaches an endosomal/lysosomal compartment where it subsequently is degraded. The analysis of regions within the viral protein that are essential for MHC class I retention revealed that a secreted form of gp40, lacking the cytoplasmic tail and the transmembrane region, still has the capacity to retain MHC class I complexes. Continuous expression of gp40 is not required for MHC class I retention. Our data indicate that the retention of MHC class I complexes in the ERGIC/cis-Golgi is triggered by gp40 and does not require the further presence of the viral protein.
Figures






References
-
- Ahn K., Gruhler, A., Galocha, B., Jones, T.R., Wiertz, E.J., Ploegh, H.L., Peterson, P.A., Yang, Y. and Früh, K. (1997) The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity, 6, 613–621. - PubMed
-
- Burgert H.-G. (1996) Subversion of the MHC class I antigen-presentation pathway by adenoviruses and herpes simplex viruses. Trends Microbiol., 4, 107–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

