Early endosomal maturation of MHC class II molecules independently of cysteine proteases and H-2DM
- PMID: 10698930
- PMCID: PMC305628
- DOI: 10.1093/emboj/19.5.882
Early endosomal maturation of MHC class II molecules independently of cysteine proteases and H-2DM
Abstract
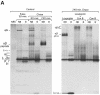
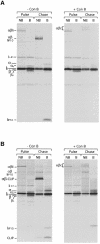
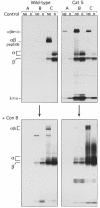
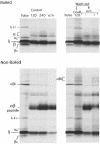
Major histocompatibility complex (MHC) class II molecules bind and present to CD4(+) T cells peptides derived from endocytosed antigens. Class II molecules associate in the endoplasmic reticulum with invariant chain (Ii), which (i) mediates the delivery of the class II-Ii complexes into the endocytic compartments where the antigenic peptides are generated; and (ii) blocks the peptide-binding site of the class II molecules until they reach their destination. Once there, Ii must be removed to allow peptide binding. The bulk of Ii-class II complexes reach late endocytic compartments where Ii is eliminated in a reaction in which the cysteine protease cathepsin S and the accessory molecule H-2DM play an essential role. Here, we here show that Ii is also eliminated in early endosomal compartments without the intervention of cysteine proteases or H-2DM. The Ii-free class II molecules generated by this alternative mechanism first bind high molecular weight polypeptides and then mature into peptide-loaded complexes.
Figures



References
-
- Avva R.R. and Cresswell, P. (1994) In vivo and in vitro formation and dissociation of HLA–DR complexes with invariant chain-derived peptides. Immunity, 1, 763–774. - PubMed
-
- Barois N., Forquet, F. and Davoust, J. (1997) Selective modulation of the major histocompatibility complex class II antigen presentation pathway following B cell receptor ligation and protein kinase C activation. J. Biol. Chem., 272, 3641–3647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

