Identification of a pocket in the PDK1 kinase domain that interacts with PIF and the C-terminal residues of PKA
- PMID: 10698939
- PMCID: PMC305637
- DOI: 10.1093/emboj/19.5.979
Identification of a pocket in the PDK1 kinase domain that interacts with PIF and the C-terminal residues of PKA
Abstract
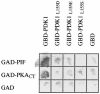
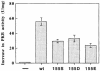
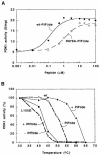
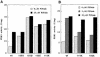
The 3-phosphoinositide-dependent protein kinase-1 (PDK1) phosphorylates and activates a number of protein kinases of the AGC subfamily. The kinase domain of PDK1 interacts with a region of protein kinase C-related kinase-2 (PRK2), termed the PDK1-interacting fragment (PIF), through a hydrophobic motif. Here we identify a hydrophobic pocket in the small lobe of the PDK1 kinase domain, separate from the ATP- and substrate-binding sites, that interacts with PIF. Mutation of residues predicted to form part of this hydrophobic pocket either abolished or significantly diminished the affinity of PDK1 for PIF. PIF increased the rate at which PDK1 phosphorylated a synthetic dodecapeptide (T308tide), corresponding to the sequences surrounding the PDK1 phosphorylation site of PKB. This peptide is a poor substrate for PDK1, but a peptide comprising T308tide fused to the PDK1-binding motif of PIF was a vastly superior substrate for PDK1. Our results suggest that the PIF-binding pocket on the kinase domain of PDK1 acts as a 'docking site', enabling it to interact with and enhance the phosphorylation of its substrates.
Figures



References
-
- Alessi D.R. and Downes, C.P. (1998) The role of PI 3-kinase in insulin action. Biochim. Biophys. Acta, 1436, 151–164. - PubMed
-
- Alessi D.R., Cohen, P., Ashworth, A., Cowley, S., Leevers, S.L. and Marshall, C.J. (1994) Assay and expression of mitogen activated protein kinase, MAP kinase and Raf. Methods Enzymol., 255, 279–290. - PubMed
-
- Alessi D.R., et al. (1997a) 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol., 7, 776–789. - PubMed
-
- Alessi D.R., James, S.R., Downes, C.P., Holmes, A.B., Gaffney, P.R., Reese, C.B. and Cohen, P. (1997b) Characterization of a 3-phospho– inositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol., 7, 261–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

