Transcription-dependent R-loop formation at mammalian class switch sequences
- PMID: 10698946
- PMCID: PMC305644
- DOI: 10.1093/emboj/19.5.1055
Transcription-dependent R-loop formation at mammalian class switch sequences
Retraction in
-
Transcription-dependent R-loop formation at mammalian class switch sequences.EMBO J. 2001 Sep 1;19(17):4855. doi: 10.1093/emboj/cdd511. EMBO J. 2001. PMID: 12134869 Free PMC article. No abstract available.
Abstract
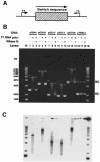
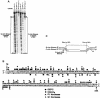
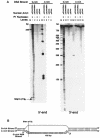
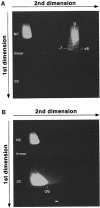
Immunoglobulin class switching is mediated by recombination between switch sequences located immediately upstream of the immunoglobulin constant heavy chain genes. Targeting of recombination to particular switch sequences is associated temporally with transcription through these regions. We recently have provided evidence for inducible and stable RNA-DNA hybrid formation at switch sequences in the mouse genome that are mechanistically important for class switching in vivo. Here, we define in vitro the precise configuration of the DNA and RNA strands within this hybrid structure at the Smicro, Sgamma3 and Sgamma2b mouse switch sequences. We find that the G-rich (non-template) DNA strand of each switch sequence is hypersensitive to probes throughout much of its length, while the C-rich (template) DNA strand is essentially resistant. These results demonstrate formation of an R-loop, whereby the G-rich RNA strand forms a stable heteroduplex with its C-rich DNA strand counterpart, and the G-rich DNA strand exists primarily in a single-stranded state. We propose that the organized structure of the R-loop is essential for targeting the class switch recombination machinery to these sequences.
Figures


Comment in
-
Findings of scientific misconduct.NIH Guide Grants Contracts (Bethesda). 2002 May 30:NOT-OD-02-051. NIH Guide Grants Contracts (Bethesda). 2002. PMID: 12449911 Free PMC article. No abstract available.
Similar articles
-
RNA:DNA complex formation upon transcription of immunoglobulin switch regions: implications for the mechanism and regulation of class switch recombination.Nucleic Acids Res. 1995 Dec 25;23(24):5006-11. doi: 10.1093/nar/23.24.5006. Nucleic Acids Res. 1995. PMID: 8559658 Free PMC article.
-
Downstream boundary of chromosomal R-loops at murine switch regions: implications for the mechanism of class switch recombination.Proc Natl Acad Sci U S A. 2006 Mar 28;103(13):5030-5. doi: 10.1073/pnas.0506548103. Epub 2006 Mar 17. Proc Natl Acad Sci U S A. 2006. PMID: 16547142 Free PMC article.
-
R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells.Nat Immunol. 2003 May;4(5):442-51. doi: 10.1038/ni919. Nat Immunol. 2003. PMID: 12679812
-
Molecular aspects of heavy-chain class switching.Crit Rev Immunol. 1989;9(3):173-200. Crit Rev Immunol. 1989. PMID: 2505810 Review.
-
Ig class switching: targeting the recombinational mechanism.Curr Opin Immunol. 2006 Jun;18(3):249-54. doi: 10.1016/j.coi.2006.03.016. Epub 2006 Apr 17. Curr Opin Immunol. 2006. PMID: 16616473 Review.
Cited by
-
Genome-wide mapping reveals conserved and diverged R-loop activities in the unusual genetic landscape of the African trypanosome genome.Nucleic Acids Res. 2018 Dec 14;46(22):11789-11805. doi: 10.1093/nar/gky928. Nucleic Acids Res. 2018. PMID: 30304482 Free PMC article.
-
Transcription-dependent R-loop formation at mammalian class switch sequences.EMBO J. 2001 Sep 1;19(17):4855. doi: 10.1093/emboj/cdd511. EMBO J. 2001. PMID: 12134869 Free PMC article. No abstract available.
-
Human activation-induced cytidine deaminase causes transcription-dependent, strand-biased C to U deaminations.Nucleic Acids Res. 2003 Jun 15;31(12):2990-4. doi: 10.1093/nar/gkg464. Nucleic Acids Res. 2003. PMID: 12799424 Free PMC article.
-
Preferential cleavage of plasmid-based R-loops and D-loops by Drosophila topoisomerase IIIbeta.Proc Natl Acad Sci U S A. 2002 Jun 11;99(12):7974-9. doi: 10.1073/pnas.122007999. Epub 2002 Jun 4. Proc Natl Acad Sci U S A. 2002. PMID: 12048241 Free PMC article.
-
RNA:DNA hybrids in the human genome have distinctive nucleotide characteristics, chromatin composition, and transcriptional relationships.Epigenetics Chromatin. 2015 Nov 16;8:46. doi: 10.1186/s13072-015-0040-6. eCollection 2015. Epigenetics Chromatin. 2015. PMID: 26579211 Free PMC article.
References
-
- Baker T.A. and Kornberg, A. (1988) Transcriptional activation of initiation of replication from the E.coli chromosomal origin: an RNA–DNA hybrid near oriC. Cell, 55, 113–123. - PubMed
-
- Carles-Kinch K. and Kreuzer, K.N. (1997) RNA–DNA hybrid formation at a bacteriophage T4 replication origin. J. Mol. Biol., 266, 915–926. - PubMed
-
- Champoux J.J. and McConaughy, B.L. (1975) Priming of superhelical SV40 DNA by E.coli RNA polymerase for in vitro DNA synthesis. Biochemistry, 14, 307–316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

