Quantitative and cost comparison of ultrasensitive human immunodeficiency virus type 1 RNA viral load assays: Bayer bDNA quantiplex versions 3.0 and 2.0 and Roche PCR Amplicor monitor version 1.5
- PMID: 10699005
- PMCID: PMC86352
- DOI: 10.1128/JCM.38.3.1113-1120.2000
Quantitative and cost comparison of ultrasensitive human immunodeficiency virus type 1 RNA viral load assays: Bayer bDNA quantiplex versions 3.0 and 2.0 and Roche PCR Amplicor monitor version 1.5
Abstract
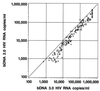
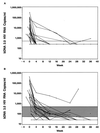
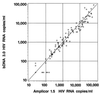
Quantification of human immunodeficiency virus type 1 (HIV-1) RNA as a measure of viral load has greatly improved the monitoring of therapies for infected individuals. With the significant reductions in viral load now observed in individuals treated with highly active anti-retroviral therapy (HAART), viral load assays have been adapted to achieve greater sensitivity. Two commercially available ultrasensitive assays, the Bayer Quantiplex HIV-1 bDNA version 3.0 (bDNA 3.0) assay and the Roche Amplicor HIV-1 Monitor Ultrasensitive version 1.5 (Amplicor 1.5) assay, are now being used to monitor HIV-1-infected individuals. Both of these ultrasensitive assays have a reported lower limit of 50 HIV-1 RNA copies/ml and were developed from corresponding older generation assays with lower limits of 400 to 500 copies/ml. However, the comparability of viral load data generated by these ultrasensitive assays and the relative costs of labor, disposables, and biohazardous wastes were not determined in most cases. In this study, we used matched clinical plasma samples to compare the quantification of the newer bDNA 3.0 assay with that of the older bDNA 2.0 assay and to compare the quantification and costs of the bDNA 3.0 assay and the Amplicor 1.5 assay. We found that quantification by the bDNA 3.0 assay was approximately twofold higher than that by the bDNA 2.0 assay and was highly correlated to that by the Amplicor 1.5 assay. Moreover, cost analysis based on labor, disposables, and biohazardous wastes showed significant savings with the bDNA 3.0 assay as compared to the costs of the Amplicor 1.5 assay.
Figures
References
-
- Brambilla D, Leung S, Lew J, Todd J, Herman S, Cronin M, Shapiro D E, Bremer J, Hanson C, Hillyer G V, McSherry G D, Sperling R S, Coombs R W, Reichelderfer P S. Absolute copy number and relative change in determinations of human immunodeficiency virus type 1 RNA in plasma: effect of an external standard on kit comparisons. J Clin Microbiol. 1998;36:311–314. - PMC - PubMed
-
- Carpenter C C, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy for HIV infection in 1998: updated recommendations of the International AIDS Society-USA Panel. JAMA. 1998;280:78–86. - PubMed
-
- Collins M L, Irvine B, Tyner D, Fine E, Zayati C, Chang C-A, Horn T, Ahle D, Detmer J, Shen L-P, Kolberg J, Bushnell S, Urdea M S, Ho D D. A branched DNA signal amplification assay for quantification of nucleic acid targets below 100 molecules/ml. Nucleic Acids Res. 1997;25:2979–2984. - PMC - PubMed
-
- Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

