Paradoxical block of the Na+-Ca2+ exchanger by extracellular protons in guinea-pig ventricular myocytes
- PMID: 10699080
- PMCID: PMC2269805
- DOI: 10.1111/j.1469-7793.2000.t01-1-00353.x
Paradoxical block of the Na+-Ca2+ exchanger by extracellular protons in guinea-pig ventricular myocytes
Abstract
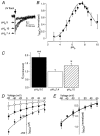
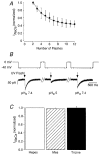
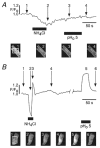
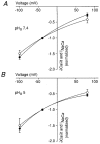
1. The Na+-Ca2+ exchange is a major pathway for removal of cytosolic Ca2+ in cardiac myocytes. It is known to be inhibited by changes of intracellular pH that may occur, for example, during ischaemia. In the present study, we examined whether extracellular protons (pHo) can also affect the cardiac exchange. 2. Na+-Ca2+ exchange currents (INa-Ca) were recorded from single adult guinea-pig ventricular myocytes in the whole-cell voltage-clamp configuration while [Ca2+]i was simultaneously imaged with fluo-3 and a laser-scanning confocal microscope. To activate INa-Ca, intracellular Ca2+ concentration jumps were generated by laser flash photolysis of caged Ca2+ (DM-nitrophen). 3. Exposure of the cell to moderately and extremely acidic conditions (pHo 6 and 4) was accompanied by a decrease of the peak INa-Ca to 70 % and less than 10 %, respectively. The peak INa-Ca was also inhibited to about 45 % of its initial value by increasing pHo to 10. The largest INa-Ca was found at pHo approximately 7.6. 4. Simultaneous measurements of [Ca2+]i and INa-Ca during partial proton block of the Na+-Ca2+ exchanger revealed that the exchange current was more inhibited by acidic pHo than the rate of Ca2+ transport. This observation is consistent with a change in the electrogenicity of the Na+-Ca2+ exchange cycle after protonation of the transporter. 5. We conclude that both extracellular alkalinization and acidification affect the Na+-Ca2+ exchanger during changes of pHo that may be present under pathophysiological conditions. During both extreme acidification or alkalinization the Na+-Ca2+ exchanger is strongly inhibited, suggesting that extracellular protons may interact with the Na+-Ca2+ exchanger at multiple sites. In addition, the electrogenicity and stoichiometry of the Na+-Ca2+ exchange may be modified by extracellular protons.
Figures


Similar articles
-
The mechanism by which cytoplasmic protons inhibit the sodium-calcium exchanger in guinea-pig heart cells.J Physiol. 1993 Jul;466:481-99. J Physiol. 1993. PMID: 8410703 Free PMC article.
-
Stoichiometry of Na+-Ca2+ exchange in inside-out patches excised from guinea-pig ventricular myocytes.J Physiol. 2000 Mar 1;523 Pt 2(Pt 2):339-51. doi: 10.1111/j.1469-7793.2000.t01-2-00339.x. J Physiol. 2000. PMID: 10699079 Free PMC article.
-
Interaction of the Na+-K+ pump and Na+-Ca2+ exchange via [Na+]i in a restricted space of guinea-pig ventricular cells.J Physiol. 1998 Jun 1;509 ( Pt 2)(Pt 2):457-70. doi: 10.1111/j.1469-7793.1998.457bn.x. J Physiol. 1998. PMID: 9575295 Free PMC article.
-
Na-Ca exchange and Ca fluxes during contraction and relaxation in mammalian ventricular muscle.Ann N Y Acad Sci. 1996 Apr 15;779:430-42. doi: 10.1111/j.1749-6632.1996.tb44818.x. Ann N Y Acad Sci. 1996. PMID: 8659859 Review.
-
Forefront of Na+/Ca2+ exchanger studies: stoichiometry of cardiac Na+/Ca2+ exchanger; 3:1 or 4:1?J Pharmacol Sci. 2004 Sep;96(1):15-8. doi: 10.1254/jphs.fmj04002x3. Epub 2004 Sep 10. J Pharmacol Sci. 2004. PMID: 15359081 Review.
Cited by
-
The Ca2+-ATPase pump facilitates bidirectional proton transport across the sarco/endoplasmic reticulum.Mol Biosyst. 2017 Mar 28;13(4):633-637. doi: 10.1039/c7mb00065k. Mol Biosyst. 2017. PMID: 28290590 Free PMC article. Review.
-
Influence of pH on Ca²⁺ current and its control of electrical and Ca²⁺ signaling in ventricular myocytes.J Gen Physiol. 2011 Nov;138(5):537-59. doi: 10.1085/jgp.201110658. J Gen Physiol. 2011. PMID: 22042988 Free PMC article.
-
Cardiac pacemaker cell failure with preserved I(f), I(CaL), and I(Kr): a lesson about pacemaker function learned from ischemia-induced bradycardia.J Mol Cell Cardiol. 2007 Feb;42(2):289-94. doi: 10.1016/j.yjmcc.2006.11.009. Epub 2006 Dec 22. J Mol Cell Cardiol. 2007. PMID: 17188292 Free PMC article. No abstract available.
-
The mechanisms of potassium loss in acute myocardial ischemia: New insights from computational simulations.Front Physiol. 2023 Feb 27;14:1074160. doi: 10.3389/fphys.2023.1074160. eCollection 2023. Front Physiol. 2023. PMID: 36923288 Free PMC article.
-
Mechanisms of Na+-Ca2+ exchange inhibition by amphiphiles in cardiac myocytes: importance of transbilayer movement.J Membr Biol. 2004 Apr 1;198(3):159-75. doi: 10.1007/s00232-004-0668-9. J Membr Biol. 2004. PMID: 15216417
References
-
- Baazov D, Wang X, Khananshvili D. Time-resolved monitoring of electrogenic Na+-Ca2+ exchange in isolated cardiac sarcolemmal vesicles by using rapid-response fluorescence probe. Biochemistry. 1999;38:1435–1445. - PubMed
-
- Baker PF, Macnaughton PA. Selective inhibition of the Ca-dependent Na efflux from intact squid axons by a fall in intracellular pH. The Journal of Physiology. 1977;269:78–79P. - PubMed
-
- Barry WH, Bridge JH. Intracellular calcium homeostasis in cardiac myocytes. Circulation. 1993;87:1806–1815. - PubMed
-
- Bassani RA, Bassani JWM, Bers DM. Relaxation in ferret ventricular myocytes: role of the sarcolemmal Ca-ATPase. Pflügers Archiv. 1995;430:573–578. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

