Ca(2+) transients and Ca(2+) waves in purkinje cells : role in action potential initiation
- PMID: 10700450
- PMCID: PMC4289140
- DOI: 10.1161/01.res.86.4.448
Ca(2+) transients and Ca(2+) waves in purkinje cells : role in action potential initiation
Abstract
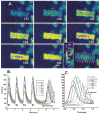
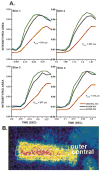
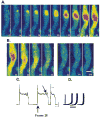
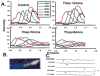
Purkinje cells contain sarcoplasmic reticulum (SR) directly under the surface membrane, are devoid of t-tubuli, and are packed with myofibrils surrounded by central SR. Several studies have reported that electrical excitation induces a biphasic Ca(2+) transient in Purkinje fiber bundles. We determined the nature of the biphasic Ca(2+) transient in aggregates of Purkinje cells. Aggregates (n=12) were dispersed from the subendocardial Purkinje fiber network of normal canine left ventricle, loaded with Fluo-3/AM, and studied in normal Tyrode's solution (24 degrees C). Membrane action potentials were recorded with fine-tipped microelectrodes, and spatial and temporal changes in [Ca(2+)](i) were obtained from fluorescent images with an epifluorescent microscope (x20; Nikon). Electrical stimulation elicited an action potential as well as a sudden increase in fluorescence (L(0)) compared with resting levels. This was followed by a further increase in fluorescence (L(1)) along the edges of the cells. Fluorescence then progressed toward the Purkinje cell core (velocity of propagation 180 to 313 microm/s). In 62% of the aggregates, initial fluorescent changes of L(0) were followed by focally arising Ca(2+) waves (L(2)), which propagated at 158+/-14 microm/s (n=13). Spontaneous Ca(2+) waves (L(2)*) propagated like L(2) (164+/-10 microm/s) occurred between stimuli and caused slow membrane depolarization; 28% of L(2)* elicited action potentials. Both spontaneous Ca(2+) wave propagation and resulting membrane depolarization were thapsigargin sensitive. Early afterdepolarizations were not accompanied by Ca(2+) waves. Action potentials in Purkinje aggregates induced a rapid rise of Ca(2+) through I(CaL) and release from a subsarcolemmal compartment (L(0)). Ca(2+) release during L(0) either induced further Ca(2+) release, which propagated toward the cell core (L(1)), or initiated Ca(2+) release from small regions and caused L(2) Ca(2+) waves, which propagated throughout the aggregate. Spontaneous Ca(2+) waves (L(2)*) induce action potentials.
Figures

References
-
- Wier WG, Isenberg G. Intracellular Ca transients in voltage clamped cardiac Purkinje fibers. Pflugers Arch. 1982;392:284–290. - PubMed
-
- Boyden PA, Albala A, Dresdner K. Electrophysiology and ultrastructure of canine subendocardial Purkinje cells isolated from control and 24 hour infarcted hearts. Circ Res. 1989;65:955–970. - PubMed
-
- Hatem SN, Benardeau A, Rucker-Martin C, Marty I, de Chamisso P, Villaz M, Mercadier JJ. Different compartments of sarcoplasmic reticulum participate in the excitation-contraction coupling process in human atrial myocytes. Circ Res. 1997;80:345–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

