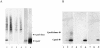Fig 2.
Detection of chaperonin 10 (Cpn10) in complex protein mixtures by immunoprecipitation and immunoblotting. Representative examples of at least 2 separate experiments are shown. (A) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis of lysate from ∼107 surface iodinated MOLT4 human T-leukemia cells precipitated with 1.5 μg of the anti-Cpn10 antibodies α1–101 (lane 1), α1–28 (lane 2), α77–101 (lane 3), the control antibody antiovalbumin (lane 4), and α1–101 after pretreatment of lysate with antiovalbumin (lane 5). In addition, 125I-Cpn10 (∼20 pg; lane 6) was analyzed in parallel. MOLT 4 cells were grown in RPMI (GibcoBRL, Rockville, MD) and 10% fetal bovine serum (FBS), harvested in the logarithmic growth phase, and washed twice with RPMI. Four samples of 3 × 107 cells were each surface iodinated, lysed, and immunoprecipitated as follows: Cells were resuspended in 0.1 mL of Hanks buffered salt solution (HBSS) and 0.1 mL of 0.1 M sodium phosphate buffer (pH, 7.4). Five Iodobeads (Pierce; rinsed thoroughly with HBSS) and 1.2 mCi carrierfree Na125I (Amersham Life Science) were then added, and the cells were shaken gently at r/t for 15 minutes. Cells were next transferred to a fresh tube, and 500 nmol mandelic acid was added as an iodine scavenger. After standing on ice for approximately 5 minutes, cells were washed twice with ice-cold RPMI and 0.01% sodium azide and then lysed (2 hours at 4°C) with 1 mL of ice-cold lysis buffer (0.01 M sodium phosphate buffer [pH, 7.4], 1% Triton X-100, 5 mM MgCl2, 0.01% sodium azide containing 10 μg/mL each of phenylmethylsulfonylflouride (PMSF), pepstatin, and leupeptin). After removal of cellular debris by centrifugation, lysates were pooled, precleared with 400 μL of ProteinA-Sepharose (Pharmacia Biotechnology, Piscataway, NY), and divided into 12 equal portions. Each portion was precipitated (overnight at 4°C) with 1.5 μg of either the antibodies described previously or (data not shown) 1 of the anti-Cpn10 antibodies (α1–11, α33–44, α29–56, or α57–76; see Table 1), followed by 20 μL of ProteinA-Sepharose. In a further experiment, lysate from 107 iodinated cells was precleared with 40μL of ProteinA-Sepharose and 5 μg of antiovalbumin, then precipitated with 1.5 μg of α1–101. Precipitated material was analyzed using SDS-PAGE under reducing conditions with 10%–20%, precast Tris-Tricine gels (Novex, San Diego, CA, USA). In addition, 125I-Cpn10 (∼20 pg, iodinated using the Iodogen technique) was analyzed in parallel. Protein was visualized by staining with Coomassie Blue, and radiolabeled material was visualized by autoradiography (BIOMAXTM MS; Eastman Kodak, Rochester, NY, USA; 8 day exposure to −80°C). (B) Purified recombinant Cpn10 in amounts of 0.5 ng (lanes 1, 4, and 7), 5 ng (lanes 2, 5, and 8), and 50 ng (lanes 3, 6, and 9) was diluted in normal human serum (dilution, 1:20) and probed with α77–101 (lanes 1–3), α1–28 (lanes 4–6), and α1–101 (lanes 7–9). Samples (10 μL) were separated by SDS-PAGE as in part A and electroblotted onto Immobilon P membranes (Millipore Corporation, Bedford, MA, USA) using a NaHCO3/Na2CO3 buffer system (Dunn et al 1986; 60 V for 1 hour at 4°C). Membranes were probed with anti-Cpn10 antibodies (1 μg/mL, 1 hour, r/t) as described previously and with other anti-Cpn10 antibodies as in part A (data not shown), followed by peroxidase-linked donkey antirabbit immunoglobulin (1/2000; Amersham Life Science, Amersham, UK) and ECL™ detection reagents (Amersham) according to the manufacturer's instructions. In a control experiment, primary antibody was omitted (data not shown). BIOMAX ML film (Eastman Kodak) was exposed to membranes for 30 seconds