A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma
- PMID: 10702402
- PMCID: PMC1876857
- DOI: 10.1016/S0002-9440(10)64954-1
A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma
Abstract
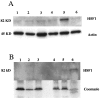
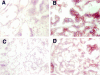
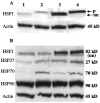
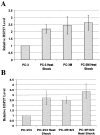
A search for differentially expressed genes in a pair of nonmetastatic (PC-3) versus metastatic variant (PC-3M) human prostate carcinoma cell lines led to identification of the human heat shock factor (HSF1) as an overexpressed gene product in PC-3M cells. Analysis of primary prostate cancer specimens indicated that HSF1 is generally up-regulated in most of the malignant prostate epithelial cells relative to the normal prostate cells. Among the known effectors of HSF1 action, constitutive levels of HSP70 and HSP90 are not significantly altered by the naturally elevated expression of HSF1 as in PC-3M cells or by transduced overexpression of HSF1 in PC-3 cells. The basal levels of HSP27 in both cases are, however, consistently increased by two- to threefold. With respect to response to heat shock, high basal concentration of HSP90 is not further enhanced in these cells, and HSP70 is up-regulated irrespective of HSF1 level. Heat shock, however, causes an increase in HSP27 when HSF1 is up-regulated, except when the expression of HSF1 is already too high. These results document for the first time that HSF1 is overexpressed in human prostate cancer cells, at least one consequence of which in the prostate cancer cell lines tested is stimulation of both basal and stress-induced expression of HSP27, an important factor in cell growth, differentiation, or apoptosis.
Figures



References
-
- Sarge KD, Zimarino V, Holm K, Wu C, Morimoto RI: Cloning and characterization of two mouse heat shock factors with distinct inducible and constitutive DNA-binding ability. Genes Dev 1991, 5:1902-1911 - PubMed
-
- Wu C: Heat shock transcription factors: structure and regulation. Ann Rev Cell Dev Biol 1995, 11:441-469 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

