Lack of endothelial nitric oxide synthase aggravates murine accelerated anti-glomerular basement membrane glomerulonephritis
- PMID: 10702405
- PMCID: PMC1876860
- DOI: 10.1016/S0002-9440(10)64957-7
Lack of endothelial nitric oxide synthase aggravates murine accelerated anti-glomerular basement membrane glomerulonephritis
Abstract
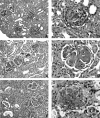
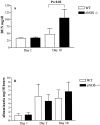
Nitric oxide (NO) radicals generated by endothelial nitric oxide synthase (eNOS) are involved in the regulation of vascular tone. In addition, NO radicals derived from eNOS inhibit platelet aggregation and leukocyte adhesion to the endothelium and, thus, may have anti-inflammatory effects. To study the role of eNOS in renal inflammation, the development of accelerated anti-glomerular basement membrane (GBM) glomerulonephritis was examined in mice lacking a functional gene for eNOS and compared with wild-type (WT) C57BL/B6j mice. WT C57BL/6j mice (n = 12) and eNOS knockout (-/-) mice (n = 12) were immunized intraperitoneally with sheep IgG (0.2 mg in complete Freund's adjuvant). At day 6.5 after immunization, mice received a single i.v. injection of sheep anti-mouse GBM (1 mg in 200 microl PBS). Mice were sacrificed at day 1 and 10 after induction of the disease. All WT mice survived until day 10, whereas 1 eNOS-/- mouse died and 2 more became moribund, requiring sacrifice. At day 10, eNOS-/- mice had higher levels of blood urea nitrogen than WT mice (P < 0.02), although proteinuria was comparable. Immunofluorescence microscopy documented similar IgG deposition in both WT and eNOS-/- mice, but eNOS-/- mice had more extensive glomerular staining for fibrin at day 10 (P < 0.007). At day 10, light microscopy demonstrated that eNOS-/- mice had more severe glomerular thrombosis (P < 0.003) and influx of neutrophils (P < 0. 006), but similar degrees of overall glomerular endocapillary hypercellularity and crescent formation. In conclusion, accelerated anti-GBM glomerulonephritis is severely aggravated in eNOS-/- mice, especially with respect to glomerular capillary thrombosis and neutrophil infiltration. These results indicate that NO radicals generated by eNOS play a protective role during renal inflammation.
Figures


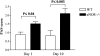

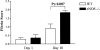

Similar articles
-
Resistance of CD28-deficient mice to autologous phase of anti-glomerular basement membrane glomerulonephritis.Clin Exp Nephrol. 2003 Jun;7(2):104-12. doi: 10.1007/s10157-003-0225-3. Clin Exp Nephrol. 2003. PMID: 14586728
-
Endothelial nitric oxide synthase-deficient mice exhibit increased susceptibility to endotoxin-induced acute renal failure.Am J Physiol Renal Physiol. 2004 Nov;287(5):F1044-8. doi: 10.1152/ajprenal.00136.2004. Am J Physiol Renal Physiol. 2004. PMID: 15475535
-
The evolution of crescentic nephritis and alveolar haemorrhage following induction of autoimmunity to glomerular basement membrane in an experimental model of Goodpasture's disease.J Pathol. 2003 May;200(1):118-29. doi: 10.1002/path.1336. J Pathol. 2003. PMID: 12692850
-
[Mice lacking the eNOS gene].Nihon Rinsho. 2004 Sep;62 Suppl 9:469-73. Nihon Rinsho. 2004. PMID: 15506429 Review. Japanese. No abstract available.
-
A protective role for endothelial nitric oxide synthase in glomerulonephritis.Kidney Int. 2002 Mar;61(3):822-5. doi: 10.1046/j.1523-1755.2002.00227.x. Kidney Int. 2002. PMID: 11849432 Review.
Cited by
-
Refinement, reduction and replacement approaches to in vivo cardiovascular research.Br J Pharmacol. 2010 Oct;161(4):749-54. doi: 10.1111/j.1476-5381.2010.00959.x. Br J Pharmacol. 2010. PMID: 20860657 Free PMC article. Review.
-
Icariside II induces rapid phosphorylation of endothelial nitric oxide synthase via multiple signaling pathways.PeerJ. 2022 Oct 25;10:e14192. doi: 10.7717/peerj.14192. eCollection 2022. PeerJ. 2022. PMID: 36312762 Free PMC article.
-
Endothelial Nitric Oxide Synthase Prevents Heparanase Induction and the Development of Proteinuria.PLoS One. 2016 Aug 9;11(8):e0160894. doi: 10.1371/journal.pone.0160894. eCollection 2016. PLoS One. 2016. PMID: 27505185 Free PMC article.
-
Repurposing Riociguat to Target a Novel Paracrine Nitric Oxide-TRPC6 Pathway to Prevent Podocyte Injury.Int J Mol Sci. 2021 Nov 19;22(22):12485. doi: 10.3390/ijms222212485. Int J Mol Sci. 2021. PMID: 34830371 Free PMC article.
-
Endothelial dysfunction exacerbates renal interstitial fibrosis through enhancing fibroblast Smad3 linker phosphorylation in the mouse obstructed kidney.PLoS One. 2013 Dec 31;8(12):e84063. doi: 10.1371/journal.pone.0084063. eCollection 2013. PLoS One. 2013. PMID: 24391884 Free PMC article.
References
-
- Huang PL, Fishman MC: Genetic analysis of nitric oxide synthase isoforms: targeted mutation in mice. J Mol Med 1996, 74:415-421 - PubMed
-
- Bachmann S, Bosse HM, Mundel P: Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol 1995, 268:F885-F898 - PubMed
-
- Granger DN, Kubes P: Nitric oxide as antiinflammatory agent. Methods Enzymol 1996, 269:434-442 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

