Endoglin expression is reduced in normal vessels but still detectable in arteriovenous malformations of patients with hereditary hemorrhagic telangiectasia type 1
- PMID: 10702408
- PMCID: PMC1876827
- DOI: 10.1016/S0002-9440(10)64960-7
Endoglin expression is reduced in normal vessels but still detectable in arteriovenous malformations of patients with hereditary hemorrhagic telangiectasia type 1
Abstract
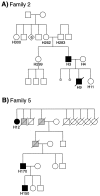
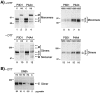
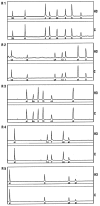
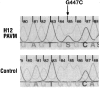
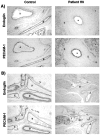
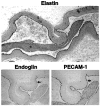
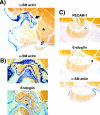
Endoglin is predominantly expressed on endothelium and is mutated in hereditary hemorrhagic telangiectasia (HHT) type 1 (HHT1). We report the analysis of endoglin in tissues of a newborn (family 2), who died of a cerebral arteriovenous malformation (CAVM), and in a lung specimen surgically resected from a 78-year-old patient (family 5), with a pulmonary AVM (PAVM). The clinically affected father of the newborn revealed a novel mutation that was absent in his parents and was identified as a duplication of exons 3 to 8, by quantitative multiplex polymerase chain reaction. The corresponding mutant protein (116-kd monomer) and the missense mutant protein (80-kd monomer) present in family 5 were detected only as transient intracellular species and were unreactive by Western blot analysis and immunostaining. Normal endoglin (90-kd monomer) was reduced by 50% on peripheral blood-activated monocytes of the HHT1 patients. When analyzed by immunostaining and densitometry, presumed normal blood vessels of the newborn lung and brain and vessels adjacent to the adult PAVM showed a 50% reduction in the endoglin/PECAM-1 ratio. A similar ratio was observed in the CAVM and PAVM, suggesting that all blood vessels of HHT1 patients express reduced endoglin in situ and that AVMs are not attributed to a focal loss of endoglin.
Figures

Similar articles
-
Mutant endoglin in hereditary hemorrhagic telangiectasia type 1 is transiently expressed intracellularly and is not a dominant negative.J Clin Invest. 1997 Nov 15;100(10):2568-79. doi: 10.1172/JCI119800. J Clin Invest. 1997. PMID: 9366572 Free PMC article.
-
Umbilical vein and placental vessels from newborns with hereditary haemorrhagic telangiectasia type 1 genotype are normal despite reduced expression of endoglin.Placenta. 2004 Feb-Mar;25(2-3):208-17. doi: 10.1016/S0143-4004(03)00181-4. Placenta. 2004. PMID: 14972453
-
Identification of hereditary hemorrhagic telangiectasia type 1 in newborns by protein expression and mutation analysis of endoglin.Pediatr Res. 2000 Jan;47(1):24-35. doi: 10.1203/00006450-200001000-00008. Pediatr Res. 2000. PMID: 10625079
-
Endoglin-deficient mice, a unique model to study hereditary hemorrhagic telangiectasia.Trends Cardiovasc Med. 2000 Oct;10(7):279-85. doi: 10.1016/s1050-1738(01)00062-7. Trends Cardiovasc Med. 2000. PMID: 11343967 Review.
-
Endovascular treatment of pulmonary and cerebral arteriovenous malformations in patients affected by hereditary haemorrhagic teleangiectasia.Curr Pharm Des. 2006;12(10):1243-8. doi: 10.2174/138161206776361237. Curr Pharm Des. 2006. PMID: 16611106 Review.
Cited by
-
Directional next-generation RNA sequencing and examination of premature termination codon mutations in endoglin/hereditary haemorrhagic telangiectasia.Mol Syndromol. 2013 Apr;4(4):184-96. doi: 10.1159/000350208. Epub 2013 Apr 11. Mol Syndromol. 2013. PMID: 23801935 Free PMC article.
-
Vascular malformations involving the female pelvis.Semin Intervent Radiol. 2008 Dec;25(4):347-60. doi: 10.1055/s-0028-1102993. Semin Intervent Radiol. 2008. PMID: 21326576 Free PMC article.
-
Minimal homozygous endothelial deletion of Eng with VEGF stimulation is sufficient to cause cerebrovascular dysplasia in the adult mouse.Cerebrovasc Dis. 2012;33(6):540-7. doi: 10.1159/000337762. Epub 2012 May 9. Cerebrovasc Dis. 2012. PMID: 22571958 Free PMC article.
-
Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease.J Med Genet. 2006 Feb;43(2):97-110. doi: 10.1136/jmg.2005.030833. Epub 2005 May 6. J Med Genet. 2006. PMID: 15879500 Free PMC article. Review.
-
Whole genome sequences discriminate hereditary hemorrhagic telangiectasia phenotypes by non-HHT deleterious DNA variation.Blood Adv. 2022 Jul 12;6(13):3956-3969. doi: 10.1182/bloodadvances.2022007136. Blood Adv. 2022. PMID: 35316832 Free PMC article.
References
-
- Guttmacher AE, Marchuk DA, White RIJ: Hereditary hemorrhagic telangiectasia. N Engl J Med 1995, 333:918-924 - PubMed
-
- Wirth JA, Pollak JS, White RIJ: Pulmonary arteriovenous malformations. Current Pulmonology and Critical Care Medicine. 1996, :pp 261-298 Mosby-Yearbook, St. Louis
-
- Braverman IM, Keh A, Jacobson BS: Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J Invest Dermatol 1990, 95:422-427 - PubMed
-
- Fayad PB: Neurologic Manifestations of Hereditary Hemorrhagic Telangiectasia. Edited by S Gilman, LB Goldstein, SG Waxman. La Jolla, CA, Neurobase, 1995
-
- Kikuchi K, Kowada M, Sasajima H: Vascular malformations of the brain in hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease). Surg Neurol 1994, 41:374-380 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

