Expression of human apolipoprotein E4 in neurons causes hyperphosphorylation of protein tau in the brains of transgenic mice
- PMID: 10702411
- PMCID: PMC1876840
- DOI: 10.1016/S0002-9440(10)64963-2
Expression of human apolipoprotein E4 in neurons causes hyperphosphorylation of protein tau in the brains of transgenic mice
Abstract
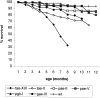
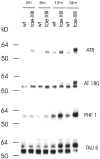
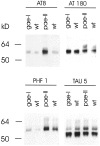
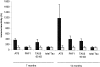
Epidemiological studies have established that the epsilon 4 allele of the ApoE gene (ApoE4) constitutes an important risk factor for Alzheimer's disease and might influence the outcome of central nervous system injury. The mechanism by which ApoE4 contributes to the development of neurodegeneration remains unknown. To test one hypothesis or mode of action of ApoE, we generated transgenic mice that overexpressed human ApoE4 in different cell types in the brain, using four distinct gene promoter constructs. Many transgenic mice expressing ApoE4 in neurons developed motor problems accompanied by muscle wasting, loss of body weight, and premature death. Overexpression of human ApoE4 in neurons resulted in hyperphosphorylation of the microtubule-associated protein tau. In three independent transgenic lines from two different promoter constructs, increased phosphorylation of protein tau was correlated with ApoE4 expression levels. Hyperphosphorylation of protein tau increased with age. In the hippocampus, astrogliosis and ubiquitin-positive inclusions were demonstrated. These findings demonstrate that expression of ApoE in neurons results in hyperphosphorylation of protein tau and suggests a role for ApoE in neuronal cytoskeletal stability and metabolism.
Figures






References
-
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA: Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261:921-923 - PubMed
-
- Strittmatter WJ, Saunders AM, Goedert M, Weisgraber KH, Dong LM, Jakes R, Huang DY, Pericak-Vance M, Schmechel D, Roses AD: Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proc Natl Acad Sci USA 1994, 91:11183-11186 - PMC - PubMed
-
- Olichney JM, Hansen LA, Galasko D, Saitoh T, Hofstetter CR, Katzman R, Thal LJ: The apolipoprotein E epsilon 4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer’s disease and Lewy body variant. Neurology 1996, 47:190-196 - PubMed
-
- Nagy Z, Esiri MM, Jobst KA, Johnston C, Litchfield S, Sim E, Smith AD: Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer’s disease. Neuroscience 1995, 69:757-761 - PubMed
-
- Gearing M, Mori H, Mirra SS: A beta-peptide length and apolipoprotein E genotype in Alzheimer’s disease. Ann Neurol 1996, 39:395-399 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

