Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing
- PMID: 10702414
- PMCID: PMC1876859
- DOI: 10.1016/S0002-9440(10)64966-8
Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing
Abstract
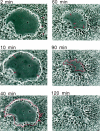
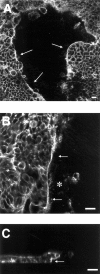
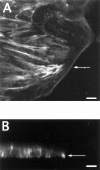
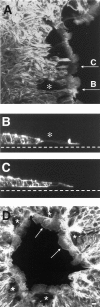
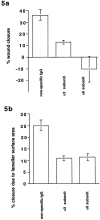
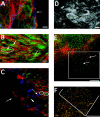
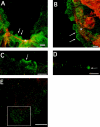
Superficial injury involving the mucosa of the gastrointestinal tract heals by a process termed restitution that involves epithelial sheet movement into the damaged area. The forces that drive epithelial sheet movement are only partially understood, although it is known to involve changes in the morphology of cells bordering the damage, such as the formation of large, flat, cytoplasmic extensions termed lamellae. We investigated the mechanism of epithelial sheet movement by following the response of the actin cytoskeleton and specific integrins (alpha6beta4, alpha6beta1, and alpha3beta1) to wounding. To model this event in vitro, monolayers of T84 cells, well-differentiated colon carcinoma cells, were damaged by aspiration and the ensuing response was analyzed by a combination of time-lapse video microscopy, fluorescence confocal microscopy and antibody inhibition assays. We show that wound healing begins with retraction of the monolayer. alpha6beta4 integrin is localized on the basal surface in structures referred to as type II hemidesmosomes that persist throughout this early stage. We hypothesize that these structures adhere to the substrate and function to retard retraction. Once retraction ceases, the wound is contracted initially by actin purse strings and then lamellae. Purse strings and lamellae produce a pulling force on surrounding cells, inducing them to flatten into the wound. In the case of lamellae, we detected actin suspension cables that appear to transduce this pulling force. As marginal cells produce lamellae, their basal type II hemidesmosomes disappear and the alpha6 integrins appear evenly distributed over lamellae surfaces. Antibodies directed against the alpha6 subunit inhibit lamellae formation, indicating that redistribution of the alpha6 integrins may contribute to the protrusion of these structures. Antibodies directed against the alpha3beta1 integrin also reduce the size and number of lamellae. This integrin's contribution to lamellae extension is most likely related to its localization at the leading edge of emerging protrusions. In summary, wounds in epithelial sheets initially retract, and then are contracted by first an actin purse string and then lamellae, both of which serve to pull the surrounding cells into the denuded area. The alpha6 integrins, particularly alpha6beta4, help contain retraction and both the alpha6 integrins and alpha3beta1 integrin contribute to lamellae formation.
Figures
References
-
- Svanes K, Ito S, Takeucchi D, Silen W: Restitution of the surface epithelium of in vitro frog gastric mucosa after damage with hyperosmolar NaCl: morphological and physiological characteristics. Gastroenterology 1982, 82:1409-1426 - PubMed
-
- Rutten MJ, Ito S: Morphology and electrophysiology of guinea pig gastric mucosal repair in vitro. Am J Physiol 1983, 244:G171-G182 - PubMed
-
- Lacy ER: Epithelial restitution in the gastrointestinal tract. J Clin Gastroenterol 1988, 10:S72-S77 - PubMed
-
- Martin P, Lewis J: Actin cables and epidermal movement in embryonic wound healing. Nature 1992, 360:179-183 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

