Reactive changes of retinal microglia during fatal murine cerebral malaria: effects of dexamethasone and experimental permeabilization of the blood-brain barrier
- PMID: 10702421
- PMCID: PMC1876828
- DOI: 10.1016/S0002-9440(10)64973-5
Reactive changes of retinal microglia during fatal murine cerebral malaria: effects of dexamethasone and experimental permeabilization of the blood-brain barrier
Abstract
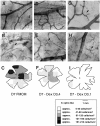
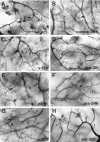
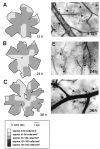
Microglial activation and redistribution toward blood vessels are some of the earliest observable events occurring within the central nervous system (CNS) during fatal murine cerebral malaria (FMCM). To investigate stimuli that might modulate microglial reactivity during FMCM we have performed two experimental manipulations and observed microglial responses in retinal whole mounts. First, to determine whether increased blood-brain barrier (BBB) permeability in the absence of the malaria parasite initiates the microglial changes, BBB function was compromised experimentally by intracarotid injection of arabinose and retinae were examined 12, 24, or 36 hours later. Second, to determine whether the immune response against the malaria parasite modulates microglial reactivity, infected mice were treated with dexamethasone before day 4 postinoculation. This treatment regime ameliorates cerebral complications without affecting parasite growth. We observed that increased BBB permeability was sufficient to elicit thickening of microglial processes and redistribution of microglia toward the vasculature, characteristic of the early stages of FMCM. However, despite the presence of plasma constituents in the CNS for up to 36 hours, microglia with amoeboid and vacuolated morphology were not observed. Dexamethasone treatment inhibited the up-regulation of alpha-D-galactose expression and reactive morphological changes in microglia during FMCM. These results suggest that disruption of the CNS milieu by entry of plasma constituents, or circulating malaria parasites in the absence of an immune response, by themselves are insufficient to induce the reactive microglial changes that are characteristic of FMCM. In addition, dexamethasone-sensitive event(s), presumably associated with immune system activation, occurring within the first few days of malaria infection are essential for the development of reactive microglia and subsequent fatal neurological complications.
Figures

Similar articles
-
Redistribution and degeneration of retinal astrocytes in experimental murine cerebral malaria: relationship to disruption of the blood-retinal barrier.Glia. 1996 Jan;16(1):51-64. doi: 10.1002/(SICI)1098-1136(199601)16:1<51::AID-GLIA6>3.0.CO;2-E. Glia. 1996. PMID: 8787773
-
Early activation of microglia in the pathogenesis of fatal murine cerebral malaria.Glia. 1997 Feb;19(2):91-103. doi: 10.1002/(sici)1098-1136(199702)19:2<91::aid-glia1>3.0.co;2-c. Glia. 1997. PMID: 9034826
-
Tumor necrosis factor-alpha expression in the brain during fatal murine cerebral malaria: evidence for production by microglia and astrocytes.Am J Pathol. 1997 Apr;150(4):1473-86. Am J Pathol. 1997. PMID: 9095002 Free PMC article.
-
Review Article: blood-brain barrier in falciparum malaria.Trop Med Int Health. 2005 Mar;10(3):285-92. doi: 10.1111/j.1365-3156.2004.01366.x. Trop Med Int Health. 2005. PMID: 15730513 Review.
-
Immunopathogenesis of cerebral malaria.Int J Parasitol. 2006 May 1;36(5):569-82. doi: 10.1016/j.ijpara.2006.02.016. Epub 2006 Mar 20. Int J Parasitol. 2006. PMID: 16678181 Review.
Cited by
-
Cytoadherence and severe malaria.Malays J Med Sci. 2012 Apr;19(2):5-18. Malays J Med Sci. 2012. PMID: 22973133 Free PMC article.
-
Cerebral malaria induces electrophysiological and neurochemical impairment in mice retinal tissue: possible effect on glutathione and glutamatergic system.Malar J. 2017 Nov 2;16(1):440. doi: 10.1186/s12936-017-2083-6. Malar J. 2017. PMID: 29096633 Free PMC article.
-
Cognitive dysfunction in mice infected with Plasmodium berghei strain ANKA.J Infect Dis. 2008 Jun 1;197(11):1621-7. doi: 10.1086/587908. J Infect Dis. 2008. PMID: 18419550 Free PMC article.
-
Glial activation and matrix metalloproteinase release in cerebral malaria.J Neurovirol. 2007;13(1):2-10. doi: 10.1080/13550280701258084. J Neurovirol. 2007. PMID: 17454443 Review.
-
Perivascular macrophages create an intravascular niche for CD8+ T cell localisation prior to the onset of fatal experimental cerebral malaria.Clin Transl Immunology. 2021 Apr 7;10(4):e1273. doi: 10.1002/cti2.1273. eCollection 2021. Clin Transl Immunology. 2021. PMID: 33854773 Free PMC article.
References
-
- Finley RW, Mackey LJ, Lambert PH: Virulent P. berghei malaria: prolonged survival and decreased cerebral pathology in T cell deficient nude mice. J Immunol 1982, 129:2213-2218 - PubMed
-
- Finley R, Weintraub J, Lewis JA, Engers HD, Zubler R, Lambert P-H: Prevention of cerebral malaria by adoptive transfer of malaria specific cultured T cells into mice infected with Plasmodium berghei. J Immunol 1983, 131:1522-1526 - PubMed
-
- Grau GE, Fajardo LF, Piguet P-F, Allet B, Lambert P-H, Vassalli P: Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 1987, 237:1210-1212 - PubMed
-
- Neill AL, Hunt NH: Pathology of fatal and resolving cerebral malaria. Parasitology 1992, 105:165-175 - PubMed
-
- Medana IM, Chan-Ling T, Hunt NH: Redistribution and degeneration of retinal astrocytes in experimental murine cerebral malaria: relationship to disruption of the blood-retinal barrier. Glia 1996, 16:51-64 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

