Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses
- PMID: 10702423
- PMCID: PMC1876862
- DOI: 10.1016/S0002-9440(10)64975-9
Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses
Abstract
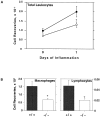
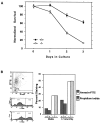
Galectin-3 is a member of a growing family of beta-galactoside-binding animal lectins. Previous studies have demonstrated a variety of biological activities for this protein in vitro, including activation of cells, modulation of cell adhesion, induction of pre-mRNA splicing, and regulation of apoptosis. To assist in fully elucidating the physiological and pathological functions of this protein, we have generated galectin-3-deficient (gal3(-/-)) mice by targeted interruption of the galectin-3 gene. Gal3(-/-) mice consistently developed fewer inflammatory cell infiltrations in the peritoneal cavities than the wild-type (gal3(+/+)) mice in response to thioglycollate broth treatment, mainly due to lower numbers of macrophages. Also, when compared to cells from gal3(+/+) mice, thioglycollate-elicited inflammatory cells from gal3(-/-) mice exhibited significantly lower levels of NF-kappaB response. In addition, dramatically different cell-spreading phenotypes were observed in cultured macrophages from the two genotypes. Whereas macrophages from gal3(+/+) mice exhibited well spread out morphology, those from gal3(-/-) mice were often spindle-shaped. Finally, we found that peritoneal macrophages from gal3(-/-) mice were more prone to undergo apoptosis than those from gal3(+/+) mice when treated with apoptotic stimuli, suggesting that expression of galectin-3 in inflammatory cells may lead to longer cell survival, thus prolonging inflammation. These results strongly support galectin-3 as a positive regulator of inflammatory responses in the peritoneal cavity.
Figures
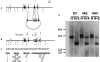



Similar articles
-
Critical role for galectin-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma.Am J Pathol. 2004 Dec;165(6):2045-53. doi: 10.1016/S0002-9440(10)63255-5. Am J Pathol. 2004. PMID: 15579447 Free PMC article.
-
Critical role of galectin-3 in phagocytosis by macrophages.J Clin Invest. 2003 Aug;112(3):389-97. doi: 10.1172/JCI17592. J Clin Invest. 2003. PMID: 12897206 Free PMC article.
-
Galectin-3 is critical for the development of the allergic inflammatory response in a mouse model of atopic dermatitis.Am J Pathol. 2009 Mar;174(3):922-31. doi: 10.2353/ajpath.2009.080500. Epub 2009 Jan 29. Am J Pathol. 2009. PMID: 19179612 Free PMC article.
-
Galectin-3 modulation of T-cell activation: mechanisms of membrane remodelling.Prog Lipid Res. 2019 Oct;76:101010. doi: 10.1016/j.plipres.2019.101010. Epub 2019 Nov 1. Prog Lipid Res. 2019. PMID: 31682868 Review.
-
Galectin-3 in metabolic disorders: mechanisms and therapeutic potential.Trends Mol Med. 2025 May;31(5):424-437. doi: 10.1016/j.molmed.2024.11.006. Epub 2024 Dec 16. Trends Mol Med. 2025. PMID: 39690058 Review.
Cited by
-
Higher galectin-3 levels are independently associated with lower anxiety in patients with risk factors for heart failure.Biopsychosoc Med. 2020 Oct 2;14:24. doi: 10.1186/s13030-020-00195-7. eCollection 2020. Biopsychosoc Med. 2020. PMID: 33024450 Free PMC article.
-
Galectins Are Central Mediators of Immune Escape in Pancreatic Ductal Adenocarcinoma.Cancers (Basel). 2022 Nov 8;14(22):5475. doi: 10.3390/cancers14225475. Cancers (Basel). 2022. PMID: 36428567 Free PMC article. Review.
-
Galectins as inflammatory mediators.Glycoconj J. 2002;19(7-9):575-81. doi: 10.1023/B:GLYC.0000014088.21242.e0. Glycoconj J. 2002. PMID: 14758082 Review.
-
Immunohistochemical localization of galectin-3 in the granulomatous lesions of paratuberculosis-infected bovine intestine.J Vet Sci. 2009 Sep;10(3):177-80. doi: 10.4142/jvs.2009.10.3.177. J Vet Sci. 2009. PMID: 19687616 Free PMC article.
-
Expression of APC protein during tongue malignant transformation in galectin-3-deficient mice challenged by the carcinogen 4-nitroquniline-n-oxide.Int J Clin Exp Pathol. 2014 May 15;7(6):3255-63. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25031746 Free PMC article.
References
-
- Barondes SH, Castronovo V, Cooper DNW, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, Leffler H, Liu F-T, Lotan R, Mercurio AM, Monsigny M, Pillai S, Poirer F, Raz A, Rigby PWJ, Rini JM, Wang JL: Galectins: A family of animal β-galactoside-binding lectins. (letter to the editor). Cell 1994, 76:597-598 - PubMed
-
- Barondes SH, Cooper DNW, Gitt MA, Leffler H: Galectins: structure and function of a large family of animal lectins. J Biol Chem 1994, 269:20807-20810 - PubMed
-
- Kasai K, Hirabayashi J: Galectins: a family of animal lectins that decipher glycocodes. J Biochem (Tokyo) 1996, 119:1-8 - PubMed
-
- Hughes RC: The galectin family of mammalian carbohydrate-binding molecules. Biochem Soc Trans 1997, 25:1194-2298 - PubMed
-
- Perillo NL, Marcus ME, Baum LG: Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med 1998, 76:402-412 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

