Evaluation of the modified ELISPOT assay for gamma interferon production in cancer patients receiving antitumor vaccines
- PMID: 10702485
- PMCID: PMC95841
- DOI: 10.1128/CDLI.7.2.145-154.2000
Evaluation of the modified ELISPOT assay for gamma interferon production in cancer patients receiving antitumor vaccines
Abstract
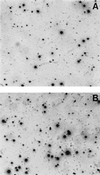
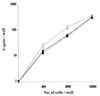
Frequencies of vaccine-responsive T-lymphocyte precursors in peripheral blood mononuclear cells (PBMC) prior to and after administration of peptide-based vaccines in patients with cancer can be measured by limiting-dilution assays (LDA) or by ELISPOT assays. We have used a modified version of the ELISPOT assay to monitor changes in the frequency of gamma interferon (IFN-gamma)-producing T cells in a population of lymphocytes responding to a relevant peptide or a nonspecific stimulator, such as phorbol myristate acetate-ionomycin. Prior to its use for monitoring of patient samples, the assay was validated and found to be comparable to the LDA performed in parallel, using tumor-reactive cytolytic T-lymphocyte (CTL) lines. The sensitivity of the ELISPOT assay was found to be 1/100,000 cells, with an interassay coefficient of variation of 15%, indicating that it could be reliably used for monitoring of changes in the frequency of IFN-gamma-secreting responder cells in noncultured or cultured lymphocyte populations. To establish that the assay is able to detect the T-cell precursor cells responsive to the vaccine, we used CD8(+) T-cell populations positively selected from PBMC of HLA-A2(+) patients with metastatic melanoma, who were treated with dendritic cell-based vaccines containing gp100, MELAN-A/MART-1, tyrosinase, and influenza virus matrix peptides. The frequency of peptide-specific responder T cells ranged from 0 to 1/2,600 before vaccination and increased by at least 1 log unit after vaccination in two patients, one of whom had a clinical response to the vaccine. However, no increases in the frequency of peptide-responsive T cells were observed in noncultured PBMC or PBMC cultured in the presence of the relevant peptides after the melanoma patients enrolled in another trial were treated with the intramuscular peptide vaccine plus MF59 adjuvant. Thus, while the ELISPOT assay was found to be readily applicable to assessments of frequencies of CTL precursors of established CTL lines and ex vivo-amplified PBMC, its usefulness for monitoring of fresh PBMC in patients with cancer was limited. In many of these patients antitumor effector T cells are present at frequencies of lower than 1/100,000 in the peripheral circulation. Serial monitoring of such patients may require prior ex vivo amplification of specific precursor cells.
Figures

References
-
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
-
- Boon T, Gajewski T F, Coulie P G. From defined human tumor antigens to effective immunization. Immunol Today. 1995;16:334–336. - PubMed
-
- Coulie P, Somville M, Lehmann F, Hainaut P, Brasseur F, Devos R, Boon T. Precursor frequency analysis of human cytolytic T lymphocytes directed against autologous melanoma cells. Int J Cancer. 1992;50:289–297. - PubMed
-
- Czerkinsky C, Anderson G, Ekre H-P, Nilsson L-A, Klareskog L, Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988;110:29–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

