Differential induction of complement fragment C5a and inflammatory cytokines during intramammary infections with Escherichia coli and Staphylococcus aureus
- PMID: 10702487
- PMCID: PMC95843
- DOI: 10.1128/CDLI.7.2.161-167.2000
Differential induction of complement fragment C5a and inflammatory cytokines during intramammary infections with Escherichia coli and Staphylococcus aureus
Abstract
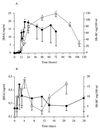
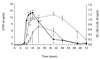
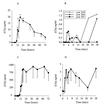
The prompt recruitment of neutrophils to the site of infection is essential for the defense of the bovine mammary gland against invading pathogens and is determinant for the outcome of the infection. Escherichia coli is known to induce clinical mastitis, characterized by an intense neutrophil recruitment leading to the eradication of the bacteria, whereas Staphylococcus aureus induces subclinical mastitis accompanied by a moderate neutrophil recruitment and the establishment of chronic mastitis. To elicit the neutrophil recruitment into the udder, inflammatory mediators must be produced after recognition of the invading pathogen. To our knowledge, those mediators have never been studied during S. aureus mastitis, although understanding of the neutrophil recruitment mechanisms could allow a better understanding of the differences in the pathogeneses elicited by E. coli and S. aureus. Therefore, we studied, at several time points, the accumulation of neutrophils and the presence of the chemoattractant complement fragment C5a and of the cytokines interleukin-1beta (IL-1beta), tumor necrosis factor alpha, and IL-8 in milk after inoculation of E. coli or S. aureus in lactating bovine udders. The low levels of C5a and the absence of cytokines in milk from S. aureus-infected cows, compared to the high levels found in milk from E. coli-infected animals, mirror the differences in the severities of the two inflammatory reactions. The cytokine deficit in milk after S. aureus inoculation in the lactating bovine mammary gland could contribute to the establishment of chronic mastitis. This result could help in the design of preventive or curative strategies against chronic mastitis.
Figures


Similar articles
-
Escherichia coli and Staphylococcus aureus elicit differential innate immune responses following intramammary infection.Clin Diagn Lab Immunol. 2004 May;11(3):463-72. doi: 10.1128/CDLI.11.3.463-472.2004. Clin Diagn Lab Immunol. 2004. PMID: 15138171 Free PMC article.
-
Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia coli.Infect Immun. 1997 Aug;65(8):3286-92. doi: 10.1128/iai.65.8.3286-3292.1997. Infect Immun. 1997. PMID: 9234788 Free PMC article.
-
Hepatic Transcriptome Analysis Identifies Divergent Pathogen-Specific Targeting-Strategies to Modulate the Innate Immune System in Response to Intramammary Infection.Front Immunol. 2020 Apr 29;11:715. doi: 10.3389/fimmu.2020.00715. eCollection 2020. Front Immunol. 2020. PMID: 32411137 Free PMC article.
-
Severity of E. coli mastitis is mainly determined by cow factors.Vet Res. 2003 Sep-Oct;34(5):521-64. doi: 10.1051/vetres:2003023. Vet Res. 2003. PMID: 14556694 Review.
-
Host-response patterns of intramammary infections in dairy cows.Vet Immunol Immunopathol. 2011 Dec 15;144(3-4):270-89. doi: 10.1016/j.vetimm.2011.08.022. Epub 2011 Sep 10. Vet Immunol Immunopathol. 2011. PMID: 21955443 Review.
Cited by
-
Stress of COVID-19, Anxiety, Economic Insecurity, and Mental Health Literacy: A Structural Equation Modeling Approach.Front Psychol. 2021 Nov 11;12:707079. doi: 10.3389/fpsyg.2021.707079. eCollection 2021. Front Psychol. 2021. PMID: 34858248 Free PMC article.
-
Transcriptomics-Based Study of Immune Genes Associated with Subclinical Mastitis in Bactrian Camels.Vet Sci. 2025 Feb 2;12(2):121. doi: 10.3390/vetsci12020121. Vet Sci. 2025. PMID: 40005880 Free PMC article.
-
Contribution of mammary epithelial cells to the immune response during early stages of a bacterial infection to Staphylococcus aureus.Vet Res. 2014 Feb 12;45(1):16. doi: 10.1186/1297-9716-45-16. Vet Res. 2014. PMID: 24521038 Free PMC article.
-
Low-level laser therapy attenuates LPS-induced rats mastitis by inhibiting polymorphonuclear neutrophil adhesion.J Vet Med Sci. 2014 Nov;76(11):1443-50. doi: 10.1292/jvms.14-0061. Epub 2014 Aug 22. J Vet Med Sci. 2014. PMID: 25452258 Free PMC article.
-
Local immunization impacts the response of dairy cows to Escherichia coli mastitis.Sci Rep. 2017 Jun 13;7(1):3441. doi: 10.1038/s41598-017-03724-7. Sci Rep. 2017. PMID: 28611405 Free PMC article.
References
-
- Anderson J C. Absence of bacterial adherence in the establishment of experimental mastitis in mice. Vet Pathol. 1978;15:770–775. - PubMed
-
- Bochner B S, Luscinskas F W, Gimbrone J, Newman W, Sterbinsky S A, Derse A C, Klunk D, Schleimer R P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991;173:1553–1557. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

