Cytoskeletal alterations in lipopolysaccharide-induced bovine vascular endothelial cell injury and its prevention by sodium arsenite
- PMID: 10702496
- PMCID: PMC95852
- DOI: 10.1128/CDLI.7.2.218-225.2000
Cytoskeletal alterations in lipopolysaccharide-induced bovine vascular endothelial cell injury and its prevention by sodium arsenite
Abstract
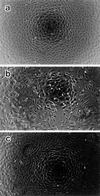
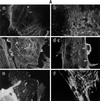
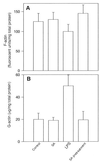
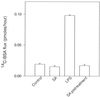
Morphological changes, especially cytoskeletal alterations, in lipopolysaccharide (LPS)-induced vascular endothelial cell injury were studied by using LPS-susceptible bovine aortic endothelial cells (BAEC). BAEC in cultures with LPS showed cell rounding, shrinking, and intercellular gap formation. In those cells, LPS caused the disorganization of actin, tubulin, and vimentin. LPS also induced a reduction in the F-actin pool and an elevation in the G-actin pool. Cytoskeletal disorganization affected transendothelial permeability across the endothelial monolayer. Pretreatment of BAEC with sodium arsenite (SA) prevented alterations in LPS-induced BAEC injury. However, posttreatment with SA had no protective effect on them. SA upregulated the expression of heat shock protein in the presence of LPS. The role of SA in prevention of LPS-induced BAEC injury is discussed.
Figures




References
-
- Alexander J S, Hechtman H B, Shepro D. Phalloidin enhances endothelial barrier function and reduces inflammatory permeability in vitro. Microvasc Res. 1988;35:308–315. - PubMed
-
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerizing-inhibiting activity. J Biol Chem. 1994;269:20780–20784. - PubMed
-
- Brandtzaeg P, Kierulf P, Gaustad P, Skulberg A, Bruun J N, Halversen S, Sorensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195–204. - PubMed
-
- Brostrom C O, Brostrom M A. Regulation of translational initiation during cellular responses to stress. Prog Nucleic Acid Res Mol Biol. 1998;58:79–125. - PubMed
-
- Burdon R H. Temperature and animal cell protein synthesis. Symp Soc Exp Biol. 1987;41:113–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

