Use of an attenuated leishmanial parasite as an immunoprophylactic and immunotherapeutic agent against murine visceral leishmaniasis
- PMID: 10702498
- PMCID: PMC95854
- DOI: 10.1128/CDLI.7.2.233-240.2000
Use of an attenuated leishmanial parasite as an immunoprophylactic and immunotherapeutic agent against murine visceral leishmaniasis
Abstract
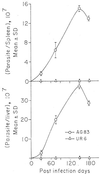
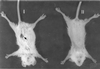
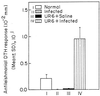
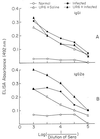
The ability of the leishmanial parasite UR6 to act as an immunoprophylactic and immunotherapeutic agent against Leishmania donovani infection in BALB/c mice was investigated. Unlike the virulent L. donovani AG83 (MOHOM/IN/1983/AG83), UR6 given through intracardiac route failed to induce visceral infection, but when it was injected subcutaneously, UR6 induced a short-lived and localized self-healing skin lesion. Priming of peritoneal macrophages with UR6 in vitro induced superoxide (O(2)(-)) generation, whereas similar experiments with virulent AG83 inhibited O(2)(-) generation. It was observed that priming of mice with either live or sonicated UR6 in the absence of any adjuvant provided strong protection against subsequent virulent challenge. Further, UR6-primed infected mice not only displayed a strong antileishmanial delayed-type hypersensitivity (DTH) response but also showed an elevated level of the serum antileishmanial immunoglobulin G2a (IgG2a) isotype, whereas infected mice failed to mount any antileishmanial DTH response and showed an elevated level of IgG1. This indicates that UR6 priming and subsequent L. donovani infection allowed the expansion of Th1 cells. Our studies indicate that UR6 has potential to be used as an immunoprophylactic and immunotherapeutic agent against experimental visceral leishmaniasis.
Figures


Similar articles
-
Immunoprophylaxis and immunotherapy against experimental visceral leishmaniasis.Vaccine. 1999 Jan 21;17(3):291-300. doi: 10.1016/s0264-410x(98)90017-2. Vaccine. 1999. PMID: 9987166
-
Immunotherapeutic Potential of Eugenol Emulsion in Experimental Visceral Leishmaniasis.PLoS Negl Trop Dis. 2016 Oct 24;10(10):e0005011. doi: 10.1371/journal.pntd.0005011. eCollection 2016 Oct. PLoS Negl Trop Dis. 2016. PMID: 27776125 Free PMC article.
-
A mixed Th1/Th2 response elicited by a liposomal formulation of Leishmania vaccine instructs Th1 responses and resistance to Leishmania donovani in susceptible BALB/c mice.Vaccine. 2004 Mar 12;22(9-10):1162-71. doi: 10.1016/j.vaccine.2003.09.030. Vaccine. 2004. PMID: 15003644
-
Serodiagnostic and immunoprophylactic potential of a 78kDa protein of Leishmania donovani of Indian origin.Med Sci Monit. 2002 Apr;8(4):BR117-22. Med Sci Monit. 2002. PMID: 11951057
-
Therapeutic immunization with radio-attenuated Leishmania parasites through i.m. route revealed protection against the experimental murine visceral leishmaniasis.Parasitol Res. 2012 Jul;111(1):361-9. doi: 10.1007/s00436-012-2847-4. Epub 2012 Mar 23. Parasitol Res. 2012. PMID: 22437790
Cited by
-
A sphingolipid rich lipid fraction isolated from attenuated Leishmania donovani promastigote induces apoptosis in mouse and human melanoma cells in vitro.Mol Cell Biochem. 2006 Oct;290(1-2):113-23. doi: 10.1007/s11010-006-9174-y. Epub 2006 May 23. Mol Cell Biochem. 2006. PMID: 16718368
-
Identification of novel Leishmania donovani antigens that help define correlates of vaccine-mediated protection in visceral leishmaniasis.PLoS One. 2009 Jun 5;4(6):e5820. doi: 10.1371/journal.pone.0005820. PLoS One. 2009. PMID: 19503834 Free PMC article.
-
Liposomal Elongation Factor-1α Triggers Effector CD4 and CD8 T Cells for Induction of Long-Lasting Protective Immunity against Visceral Leishmaniasis.Front Immunol. 2018 Jan 30;9:18. doi: 10.3389/fimmu.2018.00018. eCollection 2018. Front Immunol. 2018. PMID: 29441060 Free PMC article.
-
Therapy with radio-attenuated vaccine in experimental murine visceral leishmaniasis showed enhanced T cell and inducible nitric oxide synthase levels, suppressed tumor growth factor-beta production with higher expression of some signaling molecules.Braz J Infect Dis. 2015 Jan-Feb;19(1):36-42. doi: 10.1016/j.bjid.2014.10.009. Epub 2014 Dec 19. Braz J Infect Dis. 2015. PMID: 25532783 Free PMC article.
-
Leishmania donovani depletes labile iron pool to exploit iron uptake capacity of macrophage for its intracellular growth.Cell Microbiol. 2009 Jan;11(1):83-94. doi: 10.1111/j.1462-5822.2008.01241.x. Epub 2008 Sep 24. Cell Microbiol. 2009. PMID: 18823384 Free PMC article.
References
-
- Adams D O, Hamilton T A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. - PubMed
-
- Ali N, Afrin F. Protection of mice against visceral leishmaniasis by immunisation with promastigote antigen incorporated in liposomes. J Parasitol. 1997;83:70–75. - PubMed
-
- Basak S K, Saha B, Bhattacharya A, Roy S. Immunobiological studies on experimental visceral leishmaniasis. II. Adherent cell-mediated down-regulation of delayed-type hypersensitivity response and up-regulation of B-cell activation. Eur J Parasitol. 1992;22:2041–2045. - PubMed
-
- Berman J D. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in last 10 years. Clin Infect Dis. 1997;24:684–703. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

