PEX19 binds multiple peroxisomal membrane proteins, is predominantly cytoplasmic, and is required for peroxisome membrane synthesis
- PMID: 10704444
- PMCID: PMC2174547
- DOI: 10.1083/jcb.148.5.931
PEX19 binds multiple peroxisomal membrane proteins, is predominantly cytoplasmic, and is required for peroxisome membrane synthesis
Abstract
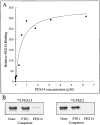
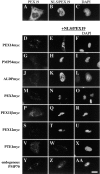
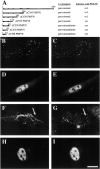
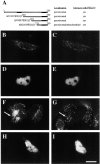
Peroxisomes are components of virtually all eukaryotic cells. While much is known about peroxisomal matrix protein import, our understanding of how peroxisomal membrane proteins (PMPs) are targeted and inserted into the peroxisome membrane is extremely limited. Here, we show that PEX19 binds a broad spectrum of PMPs, displays saturable PMP binding, and interacts with regions of PMPs required for their targeting to peroxisomes. Furthermore, mislocalization of PEX19 to the nucleus leads to nuclear accumulation of newly synthesized PMPs. At steady state, PEX19 is bimodally distributed between the cytoplasm and peroxisome, with most of the protein in the cytoplasm. We propose that PEX19 may bind newly synthesized PMPs and facilitate their insertion into the peroxisome membrane. This hypothesis is supported by the observation that the loss of PEX19 results in degradation of PMPs and/or mislocalization of PMPs to the mitochondrion.
Figures




References
-
- Adams G.A., Rose J.K. Structural requirements of a membrane-spanning domain for protein anchoring and cell surface transport. Cell. 1985;41:1007–1015. - PubMed
-
- Bjorkman J., Stetten G., Moore C.S., Gould S.J., Crane D.I. Genomic structure of PEX13, a candidate peroxisome biogenesis disorder gene. Genomics. 1998;54:521–528. - PubMed
-
- Braverman N., Dodt G., Gould S.J., Valle D. Disorders of peroxisome biogenesis. Hum. Mol. Genet. 1995;4:1791–1798. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

