Dynein-mediated cargo transport in vivo. A switch controls travel distance
- PMID: 10704445
- PMCID: PMC2174539
- DOI: 10.1083/jcb.148.5.945
Dynein-mediated cargo transport in vivo. A switch controls travel distance
Abstract
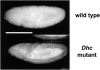
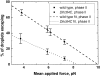
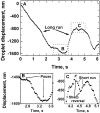
Cytoplasmic dynein is a microtubule-based motor with diverse cellular roles. Here, we use mutations in the dynein heavy chain gene to impair the motor's function, and employ biophysical measurements to demonstrate that cytoplasmic dynein is responsible for the minus end motion of bidirectionally moving lipid droplets in early Drosophila embryos. This analysis yields an estimate for the force that a single cytoplasmic dynein exerts in vivo (1.1 pN). It also allows us to quantitate dynein-mediated cargo motion in vivo, providing a framework for investigating how dynein's activity is controlled. We identify three distinct travel states whose general features also characterize plus end motion. These states are preserved in different developmental stages. We had previously provided evidence that for each travel direction, single droplets are moved by multiple motors of the same type (Welte et al. 1998). Droplet travel distances (runs) are much shorter than expected for multiple motors based on in vitro estimates of cytoplasmic dynein processivity. Therefore, we propose the existence of a process that ends runs before the motors fall off the microtubules. We find that this process acts with a constant probability per unit distance, and is typically coupled to a switch in travel direction. A process with similar properties governs plus end motion, and its regulation controls the net direction of transport.
Figures


References
-
- Block S.M., Goldstein L.S., Schnapp B.J. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990;348:348–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

