Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice
- PMID: 10704459
- PMCID: PMC2195858
- DOI: 10.1084/jem.191.5.771
Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice
Abstract
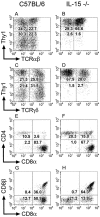
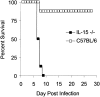
C57BL/6 mice genetically deficient in interleukin 15 (IL-15(-/-) mice) were generated by gene targeting. IL-15(-/-) mice displayed marked reductions in numbers of thymic and peripheral natural killer (NK) T cells, memory phenotype CD8(+) T cells, and distinct subpopulations of intestinal intraepithelial lymphocytes (IELs). The reduction but not absence of these populations in IL-15(-/-) mice likely reflects an important role for IL-15 for expansion and/or survival of these cells. IL-15(-/-) mice lacked NK cells, as assessed by both immunophenotyping and functional criteria, indicating an obligate role for IL-15 in the development and functional maturation of NK cells. Specific defects associated with IL-15 deficiency were reversed by in vivo administration of exogenous IL-15. Despite their immunological defects, IL-15(-/-) mice remained healthy when maintained under specific pathogen-free conditions. However, IL-15(-/-) mice are likely to have compromised host defense responses to various pathogens, as they were unable to mount a protective response to challenge with vaccinia virus. These data reveal critical roles for IL-15 in the development of specific lymphoid lineages. Moreover, the ability to rescue lymphoid defects in IL-15(-/-) mice by IL-15 administration represents a powerful means by which to further elucidate the biological roles of this cytokine.
Figures







Comment in
-
The pleiotropic functions of interleukin 15: not so interleukin 2-like after all.J Exp Med. 2000 Mar 6;191(5):753-6. doi: 10.1084/jem.191.5.753. J Exp Med. 2000. PMID: 10704456 Free PMC article. No abstract available.
References
-
- Giri J.G., Anderson D.M., Kumaki S., Park L.S., Grabstein K.H., Cosman D. IL-15, a novel T cell growth factor that shares activities and receptor components with IL-2. J. Leukoc. Biol. 1995;57:763–766. - PubMed
-
- Burton J.D., Bamford R.N., Peters C., Grant A.J., Kurys G., Goldman C.K., Brennan J., Roessler E., Waldmann T.A. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA. 1994;91:4935–4939. - PMC - PubMed
-
- Leonard W.J., Shores E.W., Love P.E. Role of the common cytokine receptor γ chain in cytokine signaling and lymphoid development. Immunol. Rev. 1995;148:97–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

