Immunotherapy with dendritic cells directed against tumor antigens shared with normal host cells results in severe autoimmune disease
- PMID: 10704461
- PMCID: PMC2195849
- DOI: 10.1084/jem.191.5.795
Immunotherapy with dendritic cells directed against tumor antigens shared with normal host cells results in severe autoimmune disease
Abstract
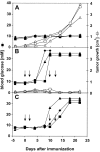
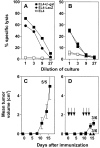
Vaccination with dendritic cells (DCs) presenting tumor antigens induces primary immune response or amplifies existing cytotoxic antitumor T cell responses. This study documents that antitumor treatment with DCs may cause severe autoimmune disease when the tumor antigens are not tumor-specific but are also expressed in peripheral nonlymphoid organs. Growing tumors with such shared tumor antigens that were, at least initially, strictly located outside of secondary lymphoid organs were successfully controlled by specific DC vaccination. However, antitumor treatment was accompanied by fatal autoimmune disease, i.e., autoimmune diabetes in transgenic mice expressing the tumor antigen also in pancreatic beta islet cells or by severe arteritis, myocarditis, and eventually dilated cardiomyopathy when arterial smooth muscle cells and cardiomyocytes expressed the transgenic tumor antigen. These results reveal the delicate balance between tumor immunity and autoimmunity and therefore point out important limitations for the use of not strictly tumor-specific antigens in antitumor vaccination with DCs.
Figures


Similar articles
-
Dendritic cells transduced with tumor-associated antigen gene elicit potent therapeutic antitumor immunity: comparison with immunodominant peptide-pulsed DCs.Oncology. 2005;68(2-3):163-70. doi: 10.1159/000086770. Epub 2005 Jul 4. Oncology. 2005. PMID: 16006753
-
Role of dendritic cells in the induction and maintenance of autoimmune diseases.Immunol Rev. 1999 Jun;169:45-54. doi: 10.1111/j.1600-065x.1999.tb01305.x. Immunol Rev. 1999. PMID: 10450507 Review.
-
Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo.J Exp Med. 1996 Jan 1;183(1):317-22. doi: 10.1084/jem.183.1.317. J Exp Med. 1996. PMID: 8551239 Free PMC article.
-
[Viral antigen induced autoimmunity: an animal model for diabetes mellitus type I].Verh Dtsch Ges Pathol. 1994;78:177-9. Verh Dtsch Ges Pathol. 1994. PMID: 7533982 German.
-
Advances in dendritic cell-based vaccine of cancer.Cancer Biother Radiopharm. 2002 Dec;17(6):601-19. doi: 10.1089/108497802320970217. Cancer Biother Radiopharm. 2002. PMID: 12537664 Review.
Cited by
-
Dendritic cells loaded with apoptotic antibody-coated tumor cells provide protective immunity against B-cell lymphoma in vivo.Blood. 2008 Feb 1;111(3):1504-11. doi: 10.1182/blood-2007-03-080507. Epub 2007 Nov 9. Blood. 2008. PMID: 17993615 Free PMC article.
-
Dendritic Cells: A Bridge between Tolerance Induction and Cancer Development in Transplantation Setting.Biomedicines. 2024 Jun 3;12(6):1240. doi: 10.3390/biomedicines12061240. Biomedicines. 2024. PMID: 38927447 Free PMC article. Review.
-
Tumor-induced endothelial cell surface heterogeneity directly affects endothelial cell escape from a cell-mediated immune response in vitro.Hum Vaccin Immunother. 2013 Jan;9(1):198-209. doi: 10.4161/hv.22828. Hum Vaccin Immunother. 2013. PMID: 23442592 Free PMC article.
-
Label-free cytokine micro- and nano-biosensing towards personalized medicine of systemic inflammatory disorders.Adv Drug Deliv Rev. 2015 Dec 1;95:90-103. doi: 10.1016/j.addr.2015.09.005. Epub 2015 Sep 25. Adv Drug Deliv Rev. 2015. PMID: 26408791 Free PMC article. Review.
-
SANTAVAC ™: A Novel Universal Antigen Composition for Developing Cancer Vaccines.Recent Pat Biotechnol. 2017;11(1):32-41. doi: 10.2174/1872208309666161130140535. Recent Pat Biotechnol. 2017. PMID: 27903220 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

