Hepatitis B virus biology
- PMID: 10704474
- PMCID: PMC98986
- DOI: 10.1128/MMBR.64.1.51-68.2000
Hepatitis B virus biology
Abstract
Hepadnaviruses (hepatitis B viruses) cause transient and chronic infections of the liver. Transient infections run a course of several months, and chronic infections are often lifelong. Chronic infections can lead to liver failure with cirrhosis and hepatocellular carcinoma. The replication strategy of these viruses has been described in great detail, but virus-host interactions leading to acute and chronic disease are still poorly understood. Studies on how the virus evades the immune response to cause prolonged transient infections with high-titer viremia and lifelong infections with an ongoing inflammation of the liver are still at an early stage, and the role of the virus in liver cancer is still elusive. The state of knowledge in this very active field is therefore reviewed with an emphasis on past accomplishments as well as goals for the future.
Figures


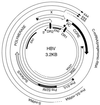

References
-
- Albritton L M, Tseng L, Scadden D, Cunningham J M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. - PubMed
-
- Aldrich C E, Coates L, Wu T T, Newbold J, Tennant B C, Summers J, Seeger C, Mason W S. In vitro infection of woodchuck hepatocytes with woodchuck hepatitis virus and ground squirrel hepatitis virus. Virology. 1989;172:247–252. - PubMed
-
- Allen M I, Deslauriers M, Andrews C W, Tipples G A, Walters K A, Tyrrell D L, Brown N, Condreay L D. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Lamivudine Clinical Investigation Group. Hepatology. 1998;27:1670–1677. - PubMed
-
- Balsano C, Avantaggiati M L, Natoli G, De M E, Will H, Perricaudet M, Levrero M. Full-length and truncated versions of the hepatitis B virus (HBV) X protein (pX) transactivate the cmyc protooncogene at the transcriptional level. Biochem Biophys Res Commun. 1991;176:985–992. - PubMed
-
- Barker L F, Chisari F V, McGrath P P, Dalgard D W, Kirschstein R L, Almeida J D, Edgington T S, Sharp D G, Peterson M R. Transmission of type B viral hepatitis to chimpanzees. J Infect Dis. 1973;127:648–652. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

