Heterogeneous conductance levels of native AMPA receptors
- PMID: 10704481
- PMCID: PMC6772487
- DOI: 10.1523/JNEUROSCI.20-06-02073.2000
Heterogeneous conductance levels of native AMPA receptors
Abstract
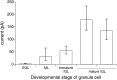
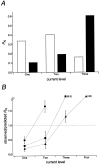
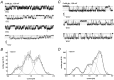
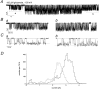
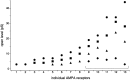
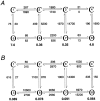
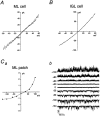
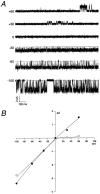
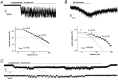
The single-channel properties of AMPA receptors can affect information processing in neurons by influencing the amplitude and kinetics of synaptic currents, yet little is known about the unitary properties of native AMPA receptors in situ. Using whole-cell and outside-out patch-clamp recordings from granule cells in acute cerebellar slices, we found that migrating granule cells begin to express AMPA receptors before they arrive in the internal granule cell layer and receive synaptic input. At saturating agonist concentrations, the open probability of channels in outside-out patches from migrating cells was very high, allowing us to identify patches that contained only one or two active channels. Analysis of the single-channel activity in these patches showed that individual AMPA receptors exhibit as many as four distinguishable conductance levels. The conductance levels observed varied substantially for different channels, although on average the values fell within the range of unitary conductances estimated previously for synaptic AMPA receptors. In contrast to patches from migrating granule cells, we rarely observed directly resolvable single-channel currents in patches excised from the somata of granule cells in the internal granular layer, even though these cells gave large AMPA receptor whole-cell currents. We did, however, detect AMPA receptors with apparent unitary conductances of <1 pS in patches from both migrating and mature granule cells. Our results suggest that granule cells express a heterogeneous population of AMPA receptors, a subset of which are segregated to postsynaptic sites after synaptogenesis.
Figures

References
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972a;145:353–398. - PubMed
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Maturation of the components of the granular layer. J Comp Neurol. 1972b;145:465–514. - PubMed
-
- Bechade C, Colin I, Kirsch J, Betz H, Triller A. Expression of glycine receptor subunits and gephyrin in cultured spinal neurons. Eur J Neurosci. 1996;8:429–435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
