cAMP-dependent plasticity at excitatory cholinergic synapses in Drosophila neurons: alterations in the memory mutant dunce
- PMID: 10704484
- PMCID: PMC6772507
- DOI: 10.1523/JNEUROSCI.20-06-02104.2000
cAMP-dependent plasticity at excitatory cholinergic synapses in Drosophila neurons: alterations in the memory mutant dunce
Abstract
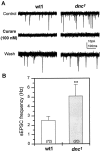
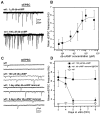
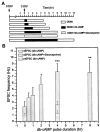
It is well known that cAMP signaling plays a role in regulating functional plasticity at central glutamatergic synapses. However, in the Drosophila CNS, where acetylcholine is thought to be a primary excitatory neurotransmitter, cellular changes in neuronal communication mediated by cAMP remain unexplored. In this study we examined the effects of elevated cAMP levels on fast excitatory cholinergic synaptic transmission in cultured embryonic Drosophila neurons. We report that chronic elevation in neuronal cAMP (in dunce neurons or wild-type neurons grown in db-cAMP) results in an increase in the frequency of cholinergic miniature EPSCs (mEPSCs). The absence of alterations in mEPSC amplitude or kinetics suggests that the locus of action is presynaptic. Furthermore, a brief exposure to db-cAMP induces two distinct changes in transmission at established cholinergic synapses in wild-type neurons: a short-term increase in the frequency of spontaneous action potential-dependent synaptic currents and a long-lasting, protein synthesis-dependent increase in the mEPSC frequency. A more persistent increase in cholinergic mEPSC frequency induced by repetitive, spaced db-cAMP exposure in wild-type neurons is absent in neurons from the memory mutant dunce. These data demonstrate that interneuronal excitatory cholinergic synapses in Drosophila, like central excitatory glutamatergic synapses in other species, are sites of cAMP-dependent plasticity. In addition, the alterations in dunce neurons suggest that cAMP-dependent plasticity at cholinergic synapses could mediate changes in neuronal communication that contribute to memory formation.
Figures



Similar articles
-
Fast excitatory synaptic transmission mediated by nicotinic acetylcholine receptors in Drosophila neurons.J Neurosci. 1999 Jul 1;19(13):5311-21. doi: 10.1523/JNEUROSCI.19-13-05311.1999. J Neurosci. 1999. PMID: 10377342 Free PMC article.
-
Synaptic depression induced by postsynaptic cAMP production in the Drosophila mushroom body calyx.J Physiol. 2018 Jun;596(12):2447-2461. doi: 10.1113/JP275799. Epub 2018 May 17. J Physiol. 2018. PMID: 29659025 Free PMC article.
-
Suppression of excitatory cholinergic synaptic transmission by Drosophila dopamine D1-like receptors.Eur J Neurosci. 2007 Nov;26(9):2417-27. doi: 10.1111/j.1460-9568.2007.05870.x. Eur J Neurosci. 2007. PMID: 17986026
-
Cellular bases of behavioral plasticity: establishing and modifying synaptic circuits in the Drosophila genetic system.J Neurobiol. 2003 Jan;54(1):254-71. doi: 10.1002/neu.10171. J Neurobiol. 2003. PMID: 12486708 Review.
-
Synaptic vesicle pools and plasticity of synaptic transmission at the Drosophila synapse.Brain Res Brain Res Rev. 2004 Dec;47(1-3):18-32. doi: 10.1016/j.brainresrev.2004.05.004. Brain Res Brain Res Rev. 2004. PMID: 15572160 Review.
Cited by
-
Preparation of neuronal cultures from midgastrula stage Drosophila embryos.J Vis Exp. 2007;(5):226. doi: 10.3791/226. Epub 2007 Jul 4. J Vis Exp. 2007. PMID: 18979024 Free PMC article.
-
GABA receptors containing Rdl subunits mediate fast inhibitory synaptic transmission in Drosophila neurons.J Neurosci. 2003 Jun 1;23(11):4625-34. doi: 10.1523/JNEUROSCI.23-11-04625.2003. J Neurosci. 2003. PMID: 12805302 Free PMC article.
-
Shal/K(v)4 channels are required for maintaining excitability during repetitive firing and normal locomotion in Drosophila.PLoS One. 2011 Jan 17;6(1):e16043. doi: 10.1371/journal.pone.0016043. PLoS One. 2011. PMID: 21264215 Free PMC article.
-
Cholinergic Homeostatic Synaptic Plasticity Drives the Progression of Aβ-Induced Changes in Neural Activity.Cell Rep. 2018 Jul 10;24(2):342-354. doi: 10.1016/j.celrep.2018.06.029. Cell Rep. 2018. PMID: 29996096 Free PMC article.
-
Pre- and Postsynaptic Role of Dopamine D2 Receptor DD2R in Drosophila Olfactory Associative Learning.Biology (Basel). 2014 Nov 21;3(4):831-45. doi: 10.3390/biology3040831. Biology (Basel). 2014. PMID: 25422852 Free PMC article.
References
-
- Abel T, Nguyen PV, Barad M, Deuel TAS, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. - PubMed
-
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. - PubMed
-
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. - PubMed
-
- Bartsch D, Casandio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. - PubMed
-
- Breer H, Sattelle DB. Molecular properties and functions of insect acetylcholine receptors. J Insect Physiol. 1987;33:771–790.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
