Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons
- PMID: 10704486
- PMCID: PMC6772506
- DOI: 10.1523/JNEUROSCI.20-06-02121.2000
Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons
Abstract
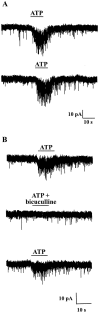
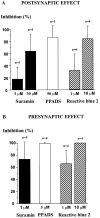
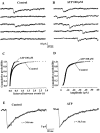
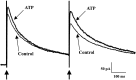
The superficial layers of the spinal cord dorsal horn (DH) express P2X2, P2X4, and P2X6 subunits entering into the formation of ionotropic (P2X) receptors for ATP. Using a culture system of laminae I-III from neonatal rat DH, we show that ATP induced a fast nonselective cation current in 38% of the neurons (postsynaptic effect). ATP also increased the frequency of miniature IPSCs (mIPSCs) mediated by GABA(A) receptors or by glycine receptors in 22 and 9%, respectively, of the neurons tested (presynaptic effect) but had no effect on glutamatergic transmission. The presynaptic effect of ATP on GABAergic transmission was not significantly affected by thapsigargin (1 microM) but was completely dependent on Ca(2+) influx. Presynaptic and postsynaptic effects were inhibited by suramin, pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid, and reactive blue and were not reproduced by uridine 5'-triphosphate (UTP) or adenosine 5'-O-(2-thiodiphosphate) (ADP-beta-S), suggesting the implication of ionotropic P2X rather than of metabotropic P2Y receptors. alphabeta-methylene-ATP (100 microM) did not reproduce the effects of ATP. ATP reversibly increased the amplitude of electrically evoked GABAergic IPSCs and reduced paired-pulse inhibition or facilitation without affecting IPSC kinetics. This effect was preferentially, but not exclusively, observed in neurons coreleasing ATP and GABA. We conclude that in cultured DH neurons, the effects of ATP are mediated by P2X receptors having a pharmacological profile dominated by the P2X2 subunit. The presynaptic receptors might underlie a modulatory action of ATP on a subset of GABAergic interneurons involved in the spinal processing of nociceptive information.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
