Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the Ras-mitogen-activated protein kinase pathway
- PMID: 10704499
- PMCID: PMC6772508
- DOI: 10.1523/JNEUROSCI.20-06-02238.2000
Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the Ras-mitogen-activated protein kinase pathway
Abstract
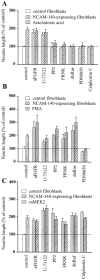
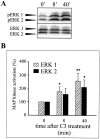
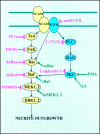
The signal transduction pathways associated with neural cell adhesion molecule (NCAM)-induced neuritogenesis are only partially characterized. We here demonstrate that NCAM-induced neurite outgrowth depends on activation of p59(fyn), focal adhesion kinase (FAK), phospholipase Cgamma (PLCgamma), protein kinase C (PKC), and the Ras-mitogen-activated protein (MAP) kinase pathway. This was done using a coculture system consisting of PC12-E2 cells grown on fibroblasts, with or without NCAM expression, allowing NCAM-NCAM interactions resulting in neurite outgrowth. PC12-E2 cells were transiently transfected with expression plasmids encoding constitutively active forms of Ras, Raf, MAP kinase kinases MEK1 and 2, dominant negative forms of Ras and Raf, and the FAK-related nonkinase. Alternatively, PC12-E2 cells were submitted to treatment with antibodies to the fibroblast growth factor (FGF) receptor, inhibitors of the nonreceptor tyrosine kinase p59(fyn), PLC, PKC and MEK and an activator of PKC, phorbol-12-myristate-13-acetate (PMA). MEK2 transfection rescued cells treated with all inhibitors. The same was found for PMA treatment, except when cells concomitantly were treated with the MEK inhibitor. Arachidonic acid rescued cells treated with antibodies to the FGF receptor or the PLC inhibitor, but not cells in which the activity of PKC, p59(fyn), FAK, Ras, or MEK was inhibited. Interaction of NCAM with a synthetic NCAM peptide ligand, known to induce neurite outgrowth, was shown to stimulate phosphorylation of the MAP kinases extracellular signal-regulated kinases ERK1 and ERK2. The MAP kinase activation was sustained, because ERK1 and ERK2 were phosphorylated in PC12-E2 cells and primary hippocampal neurons even after 24 hr of cultivation on NCAM-expressing fibroblasts. Based on these results, we propose a model of NCAM signaling involving two pathways: NCAM-Ras-MAP kinase and NCAM-FGF receptor-PLCgamma-PKC, and we propose that PKC serves as the link between the two pathways activating Raf and thereby creating the sustained activity of the MAP kinases necessary for neuronal differentiation.
Figures



References
-
- Alexinsky T, Przybyslawski J, Mileusnic R, Rose SP, Sara SJ. Antibody to day-old chick brain glycoprotein produces amnesia in adult rats. Neurobiol Learn Mem. 1997;67:14–20. - PubMed
-
- Beggs HE, Baragona SC, Hemperly JJ, Maness PF. NCAM140 interacts with the focal adhesion kinase p125 fak and the SRC-related tyrosine kinase p59fyn. J Biol Chem. 1997;272:8310–8319. - PubMed
-
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
