Late retinal progenitor cells show intrinsic limitations in the production of cell types and the kinetics of opsin synthesis
- PMID: 10704500
- PMCID: PMC6772478
- DOI: 10.1523/JNEUROSCI.20-06-02247.2000
Late retinal progenitor cells show intrinsic limitations in the production of cell types and the kinetics of opsin synthesis
Abstract
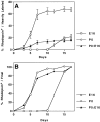
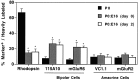
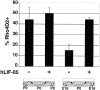
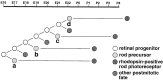
The seven major cell classes of the vertebrate neural retina arise from a pool of multipotent progenitor cells. Several studies suggest a model of retinal development in which both the environment and the progenitor cells themselves change over time (). To test this model, we used a reaggregate culture system in which a labeled population of progenitor cells from the postnatal rat retina were cultured with an excess of embryonic retinal cells. The labeled cells were then assayed for their cell fate choices and their kinetics of rod differentiation, as measured by opsin synthesis. The kinetics of opsin synthesis remained unchanged, but fewer postnatal cells adopted the rod cell fate when cultured with embryonic cells. There was an increase in the percentage of bipolar cells produced by postnatal progenitor cells, indicating a possible respecification of fate. The increase in bipolar cells could occur even after progenitor cells had completed their terminal mitoses. These alterations in cell fates appeared to be caused at least in part by a secreted factor released by the embryonic cells that requires the LIFRbeta/gp130 complex for signaling. Finally, although surrounded by 20-fold more embryonic cells, the postnatal cells did not choose to adopt any fates normally produced only by embryonic cells.
Figures
References
-
- Alexiades MR, Cepko C. Quantitative analysis of proliferation and cell cycle length during development of the rat retina. Dev Dyn. 1996;205:293–307. - PubMed
-
- Alexiades MR, Cepko CL. Subsets of retinal progenitor cells display temporally regulated and distinct biases in the fates of their progeny. Development. 1997;124:1119–1131. - PubMed
-
- Altshuler D, Cepko C. A temporally regulated, diffusible activity is required for rod photoreceptor development in vitro. Development. 1992;114:947–957. - PubMed
-
- Altshuler D, LoTurco JJR, Rush J, Cepko CL. Taurine promotes the differentiation of a vertebrate retinal cell type in vitro. Development. 1993;119:1317–1328. - PubMed
-
- Austin CP, Feldman DE, Ida JA, Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitor cells by the action of Notch. Development. 1995;121:3637–3650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
