Growth cones are not required for initial establishment of polarity or differential axon branch growth in cultured hippocampal neurons
- PMID: 10704502
- PMCID: PMC6772477
- DOI: 10.1523/JNEUROSCI.20-06-02266.2000
Growth cones are not required for initial establishment of polarity or differential axon branch growth in cultured hippocampal neurons
Abstract
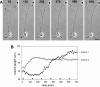
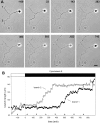
Hippocampal neurons developing in culture exhibit two types of differential, seemingly competitive, process outgrowth in the absence of external cues. During the initial acquisition of polarity, one of several equivalent undifferentiated minor neurites preferentially grows to become the axon. Once the axon has formed, it typically branches, and the branches grow differentially rather than concurrently. In axons with only two branches, growth alternates between branches. In both axon establishment and branch growth alternation, growth among sibling processes or branches must be differentially regulated. We found that elaborate and dynamic growth cones were associated with growth, whereas diminished growth cones were associated with nongrowing processes or branches. To test whether growth cones were necessary for differential growth, growth cone motility was eliminated by application of cytochalasin E. Although cytochalasin treatment before axon formation yielded longer processes overall, a similar percentage of both treated and untreated neurons had one process that grew more rapidly and became much longer than its sibling processes. Immunostaining to visualize dephospho-tau, an axonal marker, demonstrated that these single dominant processes were axons. Axons that formed in cytochalasin were thicker and showed more intense anti-tubulin staining than their sibling processes. Branched axons deprived of growth cones retained a pattern of differential growth and often included alternation. These results indicate that neither formation of a single axon nor differential growth of branches are dependent on growth cone motility and suggest that the neuron can regulate neurite elongation at sites other than at the growth cone.
Figures




Similar articles
-
An electron microscopic analysis of hippocampal neurons developing in culture: early stages in the emergence of polarity.J Neurosci. 1993 Oct;13(10):4301-15. doi: 10.1523/JNEUROSCI.13-10-04301.1993. J Neurosci. 1993. PMID: 8410189 Free PMC article.
-
Mechanical tension can specify axonal fate in hippocampal neurons.J Cell Biol. 2002 Nov 11;159(3):499-508. doi: 10.1083/jcb.200207174. Epub 2002 Nov 4. J Cell Biol. 2002. PMID: 12417580 Free PMC article.
-
Differential neurite outgrowth is required for axon specification by cultured hippocampal neurons.J Neurochem. 2012 Dec;123(6):904-10. doi: 10.1111/jnc.12001. Epub 2012 Oct 4. J Neurochem. 2012. PMID: 22928776 Free PMC article.
-
Common mechanisms underlying growth cone guidance and axon branching.J Neurobiol. 2000 Aug;44(2):145-58. J Neurobiol. 2000. PMID: 10934318 Review.
-
Axon guidance by growth cones and branches: common cytoskeletal and signaling mechanisms.Neuroscientist. 2003 Oct;9(5):343-53. doi: 10.1177/1073858403252683. Neuroscientist. 2003. PMID: 14580119 Review.
Cited by
-
Neurite outgrowth is driven by actin polymerization even in the presence of actin polymerization inhibitors.Mol Biol Cell. 2016 Sep 28;27(23):3695-704. doi: 10.1091/mbc.E16-04-0253. Online ahead of print. Mol Biol Cell. 2016. PMID: 27682586 Free PMC article.
-
Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures.Mol Cell Neurosci. 2006 Dec;33(4):345-57. doi: 10.1016/j.mcn.2006.08.005. Epub 2006 Sep 26. Mol Cell Neurosci. 2006. PMID: 17000118 Free PMC article.
-
Developmental regulation of sensory axon regeneration in the absence of growth cones.J Neurobiol. 2006 Dec;66(14):1630-45. doi: 10.1002/neu.20309. J Neurobiol. 2006. PMID: 17058187 Free PMC article.
-
NeuroRhythmics: software for analyzing time-series measurements of saltatory movements in neuronal processes.J Neurosci Methods. 2008 Aug 15;173(1):147-52. doi: 10.1016/j.jneumeth.2008.05.006. Epub 2008 May 17. J Neurosci Methods. 2008. PMID: 18572249 Free PMC article.
-
Evidence for the involvement of Tiam1 in axon formation.J Neurosci. 2001 Apr 1;21(7):2361-72. doi: 10.1523/JNEUROSCI.21-07-02361.2001. J Neurosci. 2001. PMID: 11264310 Free PMC article.
References
-
- Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323:712–715. - PubMed
-
- Bradke F, Dotti CG. Neuronal polarity: vectorial cytoplasmic flow precedes axon formation. Neuron. 1997;19:1175–1186. - PubMed
-
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
