The flathead mutation causes CNS-specific developmental abnormalities and apoptosis
- PMID: 10704505
- PMCID: PMC6772514
- DOI: 10.1523/JNEUROSCI.20-06-02295.2000
The flathead mutation causes CNS-specific developmental abnormalities and apoptosis
Abstract
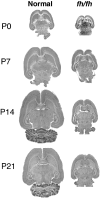
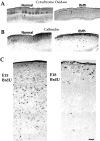
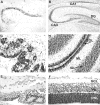
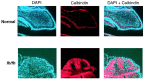
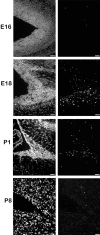
We describe a new mutation, flathead (fh), that arose spontaneously in an inbred colony of Wistar rats. The mutation is autosomal recessive, and the behavioral phenotype of fh/fh rats includes spontaneous seizures, tremor, impaired coordination, and premature death. A striking feature of the fh mutation is a dramatic reduction in brain size (40% of normal at birth). In contrast, no abnormalities are evident in the peripheral nervous system or in other tissues outside of the CNS. Although bromodeoxyuridine incorporation assays indicate that the rate of cell proliferation in the fh/fh cortex is similar to that of unaffected animals, in situ terminal deoxynucleotidyl transferase-mediated dUTP-biotin end-labeling assays reveal a dramatic increase in apoptotic cell death beginning after embryonic day 16 (E16). At E18 there is a 20-fold increase in cell death in the ventricular zone of fh/fh neocortex, and at postnatal day 1 (P1), the number of apoptotic cells is still two times that of normal. However, by P8 the extent of cell death in fh/fh is comparable to that of unaffected littermates, indicating that the reduction in brain growth is caused by abnormally high apoptosis during a discrete developmental period. Late-developing structures such as the cerebellum, neocortex, hippocampus, and retina are most severely affected by the fh mutation. Within these structures, later-generated neuronal populations are selectively depleted. Together, these results suggest that the flathead gene is essential for a developmental event required for the generation and maturation of late-born cell populations in the brain.
Figures






Similar articles
-
Aberrant apoptosis in the neurological mutant Flathead is associated with defective cytokinesis of neural progenitor cells.Brain Res Dev Brain Res. 2001 Sep 23;130(1):53-63. doi: 10.1016/s0165-3806(01)00206-1. Brain Res Dev Brain Res. 2001. PMID: 11557093
-
Characterization of seizures in the flathead rat: a new genetic model of epilepsy in early postnatal development.Epilepsia. 1999 Apr;40(4):394-400. doi: 10.1111/j.1528-1157.1999.tb00732.x. Epilepsia. 1999. PMID: 10219263
-
Histogenesis of the cerebral cortex in rat fetuses with a mutation in the Pax-6 gene.Brain Res Dev Brain Res. 2000 Mar 15;120(1):65-75. doi: 10.1016/s0165-3806(99)00187-x. Brain Res Dev Brain Res. 2000. PMID: 10727731
-
A gene essential to brain growth and development maps to the distal arm of rat chromosome 12.Neurosci Lett. 1998 Jul 17;251(1):5-8. doi: 10.1016/s0304-3940(98)00478-9. Neurosci Lett. 1998. PMID: 9714451
-
Proliferation and apoptosis in the developing human neocortex.Anat Rec. 2002 Aug 1;267(4):261-76. doi: 10.1002/ar.10100. Anat Rec. 2002. PMID: 12124904 Review.
Cited by
-
Mutations in CIT, encoding citron rho-interacting serine/threonine kinase, cause severe primary microcephaly in humans.Hum Genet. 2016 Oct;135(10):1191-7. doi: 10.1007/s00439-016-1722-2. Epub 2016 Aug 8. Hum Genet. 2016. PMID: 27503289
-
Of rings and spines: The multiple facets of Citron proteins in neural development.Small GTPases. 2020 Mar;11(2):122-130. doi: 10.1080/21541248.2017.1374325. Epub 2017 Nov 29. Small GTPases. 2020. PMID: 29185861 Free PMC article. Review.
-
Rac1 deficiency in the forebrain results in neural progenitor reduction and microcephaly.Dev Biol. 2009 Jan 1;325(1):162-70. doi: 10.1016/j.ydbio.2008.10.023. Epub 2008 Oct 31. Dev Biol. 2009. PMID: 19007770 Free PMC article.
-
Autosomal Recessive Primary Microcephaly: Not Just a Small Brain.Front Cell Dev Biol. 2022 Jan 17;9:784700. doi: 10.3389/fcell.2021.784700. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35111754 Free PMC article. Review.
-
RanBPM regulates the progression of neuronal precursors through M-phase at the surface of the neocortical ventricular zone.Dev Neurobiol. 2010 Jan;70(1):1-15. doi: 10.1002/dneu.20750. Dev Neurobiol. 2010. PMID: 19790105 Free PMC article.
References
-
- Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17:389–399. - PubMed
-
- Altman J, Bayer SA. Embryonic development of the rat cerebellum. I. Delineation of the cerebellar primordium and early cell movements. J Comp Neurol. 1985a;231:1–26. - PubMed
-
- Altman J, Bayer SA. Embryonic development of the rat cerebellum. III. Regional differences in the time of origin, migration, and settling of Purkinje cells. J Comp Neurol. 1985b;231:42–65. - PubMed
-
- Angevine JB, Sidman RL. Autoradiographic study for cell migration during histogenesis of the cerebral cortex in the mouse. Nature. 1961;192:766–768. - PubMed
-
- Bayer SA. Development of the hippocampal region in the rat. II. Morphogenesis during embryonic and early postnatal life. J Comp Neurol. 1980;190:115–134. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
