Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5
- PMID: 10706614
- PMCID: PMC16029
- DOI: 10.1073/pnas.040577797
Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5
Abstract
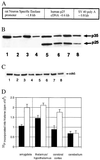
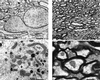
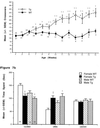
Hyperphosphorylation of microtubule-associated proteins such as tau and neurofilament may underlie the cytoskeletal abnormalities and neuronal death seen in several neurodegenerative diseases including Alzheimer's disease. One potential mechanism of microtubule-associated protein hyperphosphorylation is augmented activity of protein kinases known to associate with microtubules, such as cdk5 or GSK3beta. Here we show that tau and neurofilament are hyperphosphorylated in transgenic mice that overexpress human p25, an activator of cdk5. The p25 transgenic mice display silver-positive neurons using the Bielschowsky stain. Disturbances in neuronal cytoskeletal organization are apparent at the ultrastructural level. These changes are localized predominantly to the amygdala, thalamus/hypothalamus, and cortex. The p25 transgenic mice display increased spontaneous locomotor activity and differences from control in the elevated plus-maze test. The overexpression of an activator of cdk5 in transgenic mice results in increased cdk5 activity that is sufficient to produce hyperphosphorylation of tau and neurofilament as well as cytoskeletal disruptions reminiscent of Alzheimer's disease and other neurodegenerative diseases.
Figures




References
-
- Goedert M, Crowther R A, Spillantini M G. Neuron. 1998;21:955–958. - PubMed
-
- Imahori K, Uchida T. J Biochem. 1997;121:179–188. - PubMed
-
- Ishiguro K, Takamatsu M, Tomizawa K, Omori A, Takahashi M, Arioka M, Uchida T, Imahori K. J Biol Chem. 1992;267:10897–10901. - PubMed
-
- Hosoi T, Uchiyama M, Okumura E, Saito T, Ishiguro K, Uchida T, Okuyama A, Kishimoto T, Hisanaga S. J Biochem. 1995;117:741–740. - PubMed
-
- Wagner U, Utton M, Gallo J M, Miller C C. J Cell Sci. 1996;109:1537–1543. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

