Alphabeta protomers of Na+,K+-ATPase from microsomes of duck salt gland are mostly monomeric: formation of higher oligomers does not modify molecular activity
- PMID: 10706623
- PMCID: PMC16215
- DOI: 10.1073/pnas.97.7.3195
Alphabeta protomers of Na+,K+-ATPase from microsomes of duck salt gland are mostly monomeric: formation of higher oligomers does not modify molecular activity
Abstract
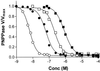
The distance that separates alphabeta protomers of the Na(+), K(+)-ATPase in microsomes and in purified membranes prepared from duck nasal salt glands was estimated by measuring fluorescence resonance energy transfer between anthroylouabain bound to a population of alphabeta protomers and either N-[7-nitrobenz-2-oxa-1, 3-diazol-4-yl]-6-aminohexyl ouabain or 5-(and-6)-carboxyfluorescein-6-aminohexyl ouabain bound to the rest. Energy transfer between probes bound in the microsomal preparation was less than in the purified membranes. The efficiency of energy transfer between anthroylouabain and N-[7-nitrobenz-2-oxa-1, 3-diazol-4-yl]-6-aminohexyl ouabain was 29.2% in the microsomes compared with 62.6% in the purified preparation. Similar results were obtained with 5-(and-6)-carboxyfluorescein-6-aminohexyl ouabain as acceptor. We calculate that either the protomer bound probes were on the average 13 A farther apart in the microsomes than in the purified membranes, or that 53% of the protomers are monomeric in the microsome preparation. Microsomes prepared in the presence of phalloidin (a toxin that binds to F actin and stabilizes the actin-based cytoskeleton) showed less quench than those prepared in its absence. The data support the hypothesis that protomers are kept apart by their association with the cytoskeleton. The turnover rate while hydrolyzing ATP is the same in the microsomal and purified preparations; higher oligomer formation has no significant effect on the enzyme reaction mechanism.
Figures

Similar articles
-
Preparation of Na+,K+-ATPase with near maximal specific activity and phosphorylation capacity: evidence that the reaction mechanism involves all of the sites.Biochemistry. 1999 Jun 8;38(23):7485-97. doi: 10.1021/bi983019b. Biochemistry. 1999. PMID: 10360946
-
Activation and inhibition of Na/K-ATPase by filipin-cholesterol complexation. A correlative biochemical and ultrastructural study on the microsomal and purified enzyme of the avian salt gland.Z Naturforsch C Biosci. 1983 Jul-Aug;38(7-8):640-63. Z Naturforsch C Biosci. 1983. PMID: 6314690
-
Ligands presumed to label high affinity and low affinity ATP binding sites do not interact in an (alpha beta)2 diprotomer in duck nasal gland Na+,K+-ATPase, nor Do the sites coexist in native enzyme.J Biol Chem. 2000 Aug 11;275(32):24512-7. doi: 10.1074/jbc.M003179200. J Biol Chem. 2000. PMID: 10831595
-
Ultrastructure of the purified and reconstituted Na/K-ATPase of the avian salt gland.Eur J Cell Biol. 1983 Jan;29(2):226-35. Eur J Cell Biol. 1983. PMID: 6299740
-
Pumping ions.Clin Exp Pharmacol Physiol. 2011 Nov;38(11):726-33. doi: 10.1111/j.1440-1681.2011.05590.x. Clin Exp Pharmacol Physiol. 2011. PMID: 21848638 Review.
Cited by
-
Mechanism of allosteric effects of ATP on the kinetics of P-type ATPases.Eur Biophys J. 2009 Dec;39(1):3-17. doi: 10.1007/s00249-009-0407-3. Epub 2009 Feb 19. Eur Biophys J. 2009. PMID: 19225774 Review.
-
Proteoliposomes in nanobiotechnology.Biophys Rev. 2012 Mar;4(1):67-81. doi: 10.1007/s12551-011-0065-4. Epub 2012 Jan 18. Biophys Rev. 2012. PMID: 28510001 Free PMC article. Review.
References
-
- Craig W S. Biochemistry. 1982;21:5707–5717. - PubMed
-
- Brotherus J R, Jacobsen L, Jørgensen P L. Biochem Biophys Acta. 1983;731:290–303. - PubMed
-
- Vilsen B, Andersen J P, Petersen J, Jørgensen P L. J Biol Chem. 1987;262:10511–10517. - PubMed
-
- Hayashi Y, Mimura K, Matsui H, Takagi T. Biochim Biophys Acta. 1989;983:217–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

