Human cytomegalovirus UL69 protein is required for efficient accumulation of infected cells in the G1 phase of the cell cycle
- PMID: 10706637
- PMCID: PMC15991
- DOI: 10.1073/pnas.050587597
Human cytomegalovirus UL69 protein is required for efficient accumulation of infected cells in the G1 phase of the cell cycle
Abstract
Human cytomegalovirus blocks cell-cycle progression in the G(1) compartment upon infection of primary human fibroblasts. The virus-coded UL69 protein can institute a G(1) block when expressed in cells in the absence of virus infection. We have constructed a cytomegalovirus mutant, TNsubUL69, that lacks the UL69 coding region. This virus grows slowly in fibroblasts, but produces a wild-type yield after an extended delay. It grows with normal kinetics in cells coinfected with a recombinant retrovirus, retroUL69, which expresses UL69 protein, demonstrating that its growth defect results from the mutation in the UL69 gene. UL69 protein is packaged within virus particles, and it was possible for us to produce two types of virus stocks. TNsubUL69(+pUL69) lacks the UL69 gene but contains UL69 protein in virus particles. It is produced by growth in fibroblasts that are coinfected with retroUL69. TNsubUL69(-pUL69) lacks the UL69 gene and protein. It is produced by growth in fibroblasts that do not contain UL69 protein. The mutant virions lacking both the UL69 gene and protein fail to induce a cell-cycle block with normal efficiency, whereas the mutant particles lacking the gene but containing the protein can institute the block. These results are consistent with the view that the UL69 protein contributes to the cytomegalovirus-induced cell-cycle block, and they suggest that UL69 protein delivered to cells within virions can induce the block without the synthesis of additional UL69 protein encoded by the infecting viral genome.
Figures
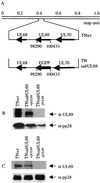
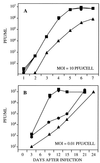

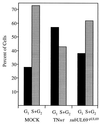
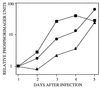

References
-
- Alfred C A, Stagno S, Pass R F, Britt W. Rev Infect Dis. 1990;12:S745–S753. - PubMed
-
- Britt W, Alfred C A. In: Fields Virology. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippincott-Raven; 1996. pp. 2493–2523.
-
- Bresnahan W A, Boldogh I, Thompson E A, Albrecht T. Virology. 1996;224:150–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

