Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells
- PMID: 10706639
- PMCID: PMC15967
- DOI: 10.1073/pnas.050588497
Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells
Abstract
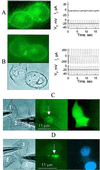
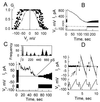
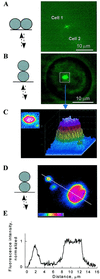
Communication-incompetent cell lines were transfected with connexin (Cx) 43 fused with enhanced green fluorescent protein (EGFP) to examine the relation between Cx distribution determined by fluorescence microscopy and electrical coupling measured at single-channel resolution in living cell pairs. Cx43-EGFP channel properties were like those of wild-type Cx43 except for reduced sensitivity to transjunctional voltage. Cx43-EGFP clustered into plaques at locations of cell-cell contact. Coupling was always absent in the absence of plaques and even in the presence of small plaques. Plaques exceeding several hundred channels always conferred coupling, but only a small fraction of channels were functional. These data indicate that clustering may be a requirement for opening of gap junction channels.
Figures

References
-
- Willecke K, Hennemann H, Dahl E, Jungbluth S, Heynkes R. Eur J Cell Biol. 1991;56:1–7. - PubMed
-
- Goodenough D A, Goliger J A, Paul D L. Annu Rev Biochem. 1996;65:475–502. - PubMed
-
- Bergoffen J, Scherer S S, Wang S, Scott M O, Bone L J, Paul D L, Chen K, Lensch M W, Chance P F, Fischbeck K H. Science. 1993;262:2039–2042. - PubMed
-
- Ionasescu V, Searby C, Ionasescu R. Hum Mol Genet. 1994;3:355–358. - PubMed
-
- Kelsell D P, Dunlop J, Stevens H P, Lench N J, Liang J N, Parry G, Mueller R F, Leigh I M. Nature (London) 1997;387:80–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

