Mengovirus and encephalomyocarditis virus poly(C) tract lengths can affect virus growth in murine cell culture
- PMID: 10708422
- PMCID: PMC111806
- DOI: 10.1128/jvi.74.7.3074-3081.2000
Mengovirus and encephalomyocarditis virus poly(C) tract lengths can affect virus growth in murine cell culture
Abstract
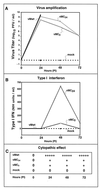
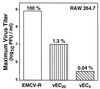
Many virulent aphthoviruses and cardioviruses have long homopolymeric poly(C) tracts in the 5' untranslated regions of their RNA genomes. A panel of genetically engineered mengo-type cardioviruses has been described which contain a variety of different poly(C) tract lengths. Studies of these viruses have shown the poly(C) tract to be dispensable for growth in HeLa cells, although the relative murine virulence of the viruses correlates directly and positively with tract length. Compared with wild-type mengovirus strain M, mutants with shortened poly(C) tracts grow poorly in mice and protectively immunize rather than kill recipient animals. In the present study, several murine cell populations were tested to determine whether, unlike HeLa cells, they allowed a differential amplification of viruses with long or short poly(C) tracts. Replication and cytopathic studies with four hematopoietically derived cell lines (CH2B, RAW 264.7, A20.J, and P815) and two murine fibroblast cell lines [L929 and L(Y)] demonstrated that several of these cell types indeed allowed differential virus replication as a function of viral poly(C) tract length. Among the most discerning of these cells, RAW 264.7 macrophages supported vigorous lytic growth of a long-tract virus, vMwt (C(44)UC(10)), but supported only substantially diminished and virtually nonlytic growth of vMC(24) (C(13)UC(10)) and vMC(0) short-tract viruses. The viral growth differences evident in all cell lines were apparent early and continuously during every cycle of virus amplification. The data suggest that poly(C) tract-dependent attenuation of mengovirus may be due in part to a viral replication defect manifest in similar hematopoietic-type cells shortly after murine infection. The characterized cultures should provide excellent tools for molecular study of poly(C) tract-mediated virulence.
Figures


References
-
- Backues K A, Hill M, Palmenberg A C, Miller C, Soike K F, Aguilar R. Genetically engineered Mengo virus vaccination of multiple captive wildlife species. J Wild Dis. 1999;35:384–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

