Virus-induced diabetes in a transgenic model: role of cross-reacting viruses and quantitation of effector T cells needed to cause disease
- PMID: 10708445
- PMCID: PMC111829
- DOI: 10.1128/jvi.74.7.3284-3292.2000
Virus-induced diabetes in a transgenic model: role of cross-reacting viruses and quantitation of effector T cells needed to cause disease
Abstract
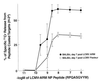
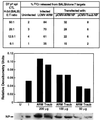
Virus-specific cytotoxic T lymphocytes (CTL) at frequencies of >1/1, 000 are sufficient to cause insulin-dependent diabetes mellitus (IDDM) in transgenic mice whose pancreatic beta cells express as "self" antigen a protein from a virus later used to initiate infection. The inability to generate sufficient effector CTL for other cross-reacting viruses that fail to cause IDDM could be mapped to point mutations in the CTL epitope or its COO(-) flanking region. These data indicate that IDDM and likely other autoimmune diseases are caused by a quantifiable number of T cells, that neither standard epidemiologic markers nor molecular analysis with nucleic acid probes reliably distinguishes between viruses that do or do not cause diabetes, and that a single-amino-acid change flanking a CTL epitope can interfere with antigen presentation and development of autoimmune disease in vivo.
Figures



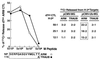

References
-
- Baekkeskov S, Hansen B, editors. Human diabetes: Genetic, environmental and autoimmune etiology. Vol. 164. Heidelberg, Germany: Springer-Verlag; 1990.
-
- Buchmeier M J, Lewicki H A, Tomori O, Oldstone M B A. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981;113:73–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

