The lymphocytic choriomeningitis virus RING protein Z associates with eukaryotic initiation factor 4E and selectively represses translation in a RING-dependent manner
- PMID: 10708446
- PMCID: PMC111830
- DOI: 10.1128/jvi.74.7.3293-3300.2000
The lymphocytic choriomeningitis virus RING protein Z associates with eukaryotic initiation factor 4E and selectively represses translation in a RING-dependent manner
Abstract
Only a few host cell proteins that associate with arenaviruses have been identified. To date, the arenavirus Z protein associates with the promyelocytic leukemia protein PML and the ribosomal P proteins. The majority of PML is present in nuclear bodies which are translocated to the cytoplasm by infection with the arenavirus, lymphocytic choriomeningitis virus (LCMV). The Z protein is a small zinc-binding RING protein with an unknown function which is required for the viral life cycle. Here, we demonstrate an association between Z and the host cell translation factor, eukaryotic initiation factor 4E (eIF-4E) in infected and transfected cells. Z's association with both ribosomal proteins and this translation factor led us to investigate whether Z could modulate host cell translation. In cell culture, Z selectively represses protein production in an eIF-4E-dependent manner. Specifically, we see reduction in cyclin D1 protein production with no effect on glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in cells transfected with Z. Previous reports indicate that cyclin D1 is sensitive to eIF-4E levels, whereas GAPDH is not. Consistent with this, we observe preferential downregulation of cyclin D1 during infection and no effect on GAPDH. Further, no changes in RNA levels were observed for cyclin D1 or GAPDH transcripts. The interaction between eIF-4E and Z may provide a mechanism for slower growth observed in infected cells and a viral strategy for establishing chronic infection.
Figures



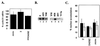

References
-
- Borden K L B. RING domains: master builders of molecular scaffolds? J Mol Biol. 2000;295:1103–1112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

