Rotavirus spike protein VP4 is present at the plasma membrane and is associated with microtubules in infected cells
- PMID: 10708448
- PMCID: PMC111832
- DOI: 10.1128/jvi.74.7.3313-3320.2000
Rotavirus spike protein VP4 is present at the plasma membrane and is associated with microtubules in infected cells
Abstract
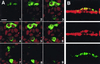
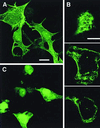
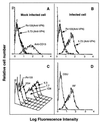
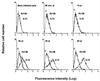
VP4 is an unglycosylated protein of the outer layer of the capsid of rotavirus. It forms spikes that project from the outer layer of mature virions, which is mainly constituted by glycoprotein VP7. VP4 has been implicated in several important functions, such as cell attachment, penetration, hemagglutination, neutralization, virulence, and host range. Previous studies indicated that VP4 is located in the space between the periphery of the viroplasm and the outside of the endoplasmic reticulum in rotavirus-infected cells. Confocal microscopy of infected MA104 monolayers, immunostained with specific monoclonal antibodies, revealed that a significant fraction of VP4 was present at the plasma membrane early after infection. Another fraction of VP4 is cytoplasmic and colocalizes with beta-tubulin. Flow cytometry analysis confirmed that at the early stage of viral infection, VP4 was present on the plasma membrane and that its N-terminal region, the VP8* subunit, was accessible to antibodies. Biotin labeling of the infected cell surface monolayer with a cell-impermeable reagent allowed the identification of the noncleaved form of VP4 that was associated with the glycoprotein VP7. The localization of VP4 was not modified in cells transfected with a plasmid allowing the expression of a fusion protein consisting of VP4 and the green fluorescent protein. The present data suggest that VP4 reaches the plasma membrane through the microtubule network and that other viral proteins are dispensable for its targeting and transport.
Figures


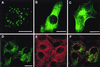
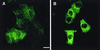
Similar articles
-
Antibodies to rotavirus outer capsid glycoprotein VP7 neutralize infectivity by inhibiting virion decapsidation.J Virol. 2002 Jul;76(13):6643-51. doi: 10.1128/jvi.76.13.6643-6651.2002. J Virol. 2002. PMID: 12050377 Free PMC article.
-
Interactions of rotavirus VP4 spike protein with the endosomal protein Rab5 and the prenylated Rab acceptor PRA1.J Virol. 2003 Jun;77(12):7041-7. doi: 10.1128/jvi.77.12.7041-7047.2003. J Virol. 2003. PMID: 12768023 Free PMC article.
-
The C Terminus of Rotavirus VP4 Protein Contains an Actin Binding Domain Which Requires Cooperation with the Coiled-Coil Domain for Actin Remodeling.J Virol. 2018 Dec 10;93(1):e01598-18. doi: 10.1128/JVI.01598-18. Print 2019 Jan 1. J Virol. 2018. PMID: 30333172 Free PMC article.
-
Actin-Dependent Nonlytic Rotavirus Exit and Infectious Virus Morphogenetic Pathway in Nonpolarized Cells.J Virol. 2018 Feb 26;92(6):e02076-17. doi: 10.1128/JVI.02076-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29263265 Free PMC article.
-
Rotavirus assembly: an alternative model that utilizes an atypical trafficking pathway.Curr Top Microbiol Immunol. 2006;309:245-61. doi: 10.1007/3-540-30773-7_9. Curr Top Microbiol Immunol. 2006. PMID: 16909902 Free PMC article. Review.
Cited by
-
Heterogeneity of Raft-type membrane microdomains associated with VP4, the rotavirus spike protein, in Caco-2 and MA 104 cells.J Virol. 2007 Feb;81(4):1610-8. doi: 10.1128/JVI.01433-06. Epub 2006 Nov 29. J Virol. 2007. PMID: 17135322 Free PMC article.
-
Rotavirus NSP4: Cell type-dependent transport kinetics to the exofacial plasma membrane and release from intact infected cells.Virol J. 2011 Jun 6;8:278. doi: 10.1186/1743-422X-8-278. Virol J. 2011. PMID: 21645398 Free PMC article.
-
Association of ebola virus matrix protein VP40 with microtubules.J Virol. 2005 Apr;79(8):4709-19. doi: 10.1128/JVI.79.8.4709-4719.2005. J Virol. 2005. PMID: 15795257 Free PMC article.
-
Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion.J Virol. 2006 Nov;80(21):10692-9. doi: 10.1128/JVI.00927-06. Epub 2006 Sep 6. J Virol. 2006. PMID: 16956954 Free PMC article.
-
Mouse hepatitis virus replicase protein complexes are translocated to sites of M protein accumulation in the ERGIC at late times of infection.Virology. 2001 Jun 20;285(1):21-9. doi: 10.1006/viro.2001.0932. Virology. 2001. PMID: 11414802 Free PMC article.
References
-
- Bellamy A R, Both G W. Molecular biology of rotaviruses. Adv Virus Res. 1990;38:1–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

