An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor
- PMID: 10708449
- PMCID: PMC111833
- DOI: 10.1128/jvi.74.7.3321-3329.2000
An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor
Abstract
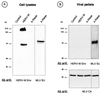
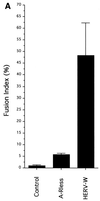
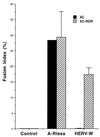
A new human endogenous retrovirus (HERV) family, termed HERV-W, was recently described (J.-L. Blond, F. Besème, L. Duret, O. Bouton, F. Bedin, H. Perron, B. Mandrand, and F. Mallet, J. Virol. 73:1175-1185, 1999). HERV-W mRNAs were found to be specifically expressed in placenta cells, and an env cDNA containing a complete open reading frame was recovered. In cell-cell fusion assays, we demonstrate here that the product of the HERV-W env gene is a highly fusogenic membrane glycoprotein. Transfection of an HERV-W Env expression vector in a panel of cell lines derived from different species resulted in formation of syncytia in primate and pig cells upon interaction with the type D mammalian retrovirus receptor. Moreover, envelope glycoproteins encoded by HERV-W were specifically detected in placenta cells, suggesting that they may play a physiological role during pregnancy and placenta formation.
Figures


References
-
- Bateman, A., F. Bullough, S. Murphy, L. Emiliusen, D. Lavillette, F.-L. Cosset, R. Cattaneo, S. J. Russell, and R. Vile. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumour growth. Cancer Res., in press. - PubMed
-
- Berlioz-Torrent C, Shacklett B L, Erdtmann L, Delamarre L, Bouchaert I, Sonigo P, Dokhelar M C, Benarous R. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J Virol. 1999;73:1350–1361. - PMC - PubMed
-
- Boeke J D, Stoye J P. Retrotransposons, endogenous retroviruses, and the evolution of retroelements. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 343–435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

